Home
ABOUT DR. VADIM JUCAUD
Dr. Vadim Jucaud is an Assistant Professor at the Terasaki Institute. Dr. Jucaud has been part of the Terasaki Laboratory since 2010, where he started his research career under the mentorship of Professor Paul I. Terasaki in the fields of histocompatibility and immunogenetics, with a particular emphasis on HLA antibodies and the Humoral Theory of Transplantation.
Dr. Jucaud has a Ph.D. in Immunology and Microbiology, specializing in transplant immunology and Human Leukocyte Antigen (HLA) immunobiology, notably in the diversity, detection, and prediction of the humoral response against HLA molecules. Dr. Jucaud is an expert in HLA immunogenetics, histocompatibility, and immunogenicity, with excellent technical skills forged by 12 years of experience in characterizing the humoral immune response in transplant patients, T and B cell immunomodulation, sensitive multiplex immunoassays, antibody cross-reactivity and immunogenic antibody epitopes, and HLA antibody/antigen interactions. Dr. Jucaud received the American Transplant Congress Young Investigator Award in 2018 for his outstanding work demonstrating the prevalence and impact of de novo donor-specific antibodies during a multicenter immunosuppression withdrawal trial in adult liver transplant recipients.
Motivated by his background and the exciting multidisciplinary research at TIBI, Dr. Jucaud established VJLabs in 2020, which proposes a unique approach to combine HLA immunobiology, organ-on-a-chip technologies, tissue engineering, biomaterials, and biosensors for developing novel immunocompetent organs-on-a-chip platforms and microphysiological systems. The long-term objective of VJLabs is to engineer innovative in vitro models recapitulating complex biological mechanisms of organ physiology and disease states to be considered by regulatory authorities as an indispensable step in accelerating drug discovery and development pipelines and promoting personalized medicine applications while reducing the need for animal model testing.
VJLABS News
Advancing Monoclonal Antibody Biomanufacturing
Dr. Vadim Jucaud in collaboration with Dr. Jing Yong Ye from the University of Texas San Antonio, has developed a new microfluidic-based biosensing platform that allows monitoring cellular secretion of monoclonal antibody in real time.
A Novel Liver Cancer-on-a-chip Model to Test Embolic Agents in vitro
Dr. Vadim Jucaud's lab at the Terasaki Institute has developed a human vascularized liver cancer-on-a-chip model to evaluate vessel remodeling and cell death in response to embolic agents.
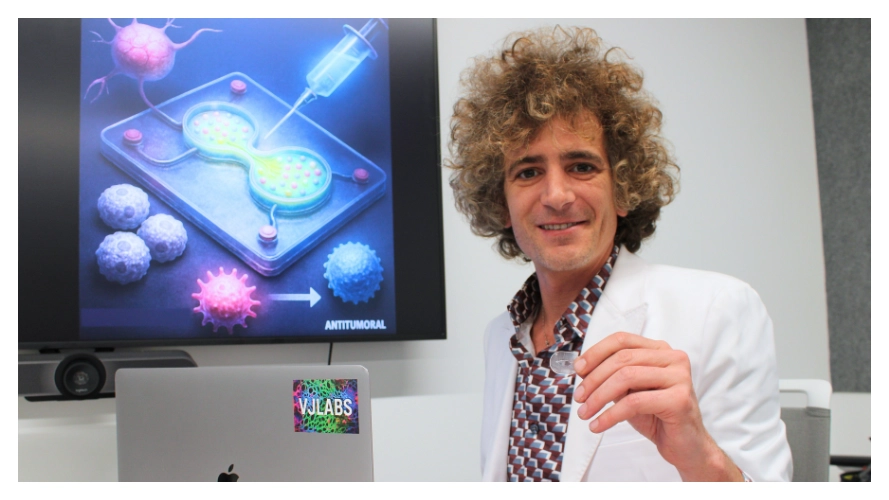
Organ-on-a-Chip Platform Allows the Testing of Cancer Vaccine in Aging Populations
Dr. Vadim Jucaud’s lab has introduced a new organ-on-a-chip platform that recapitulates age-dependent immune responses, offering a more accurate testing bed for evaluating cancer vaccine performance in older adults, the population most affected by cancer and often overlooked in traditional preclinical testing.
Remembering the Legacy of Dr. Paul I. Terasaki
In honor of Dr. Paul Terasaki’s birthday, Dr. Vadim Jucaud, one of Dr. Terasaki’s mentees, delivered an inspiring talk celebrating his mentor’s life and legacy. The presentation reflected on Dr. Terasaki’s remarkable journey and the development of the tissue-typing test, an innovation that transformed the field of transplantation. Dr. Paul Terasaki’s work has profoundly impacted countless lives, and his legacy continues to grow and thrive through the mission of the Terasaki Institute.
Dr. Vadim Jucaud recently moderated a special Fireside Chat titled “Remembering Dr. Paul I. Terasaki”, honoring the extraordinary life and legacy of Dr. Paul I. Terasaki. The discussion brought together distinguished leaders who spoke about Dr. Terasaki’s impactful work across academia, medicine, and entrepreneurship. The session featured Dr. Jerzy Kupiec-Weglinski, Paul I. Terasaki Endowed Chair of Surgery at UCLA; Dr. Jon Kobashigawa, Associate Director of the Cedars-Sinai Heart Institute; and Mr. George Ayoub, former CEO and President of One Lambda, Inc. Together, they reflected on Dr. Terasaki’s pioneering role in advancing transplantation science and fostering innovation that continues to shape modern biomedical research.
RESEARCH OVERVIEW
Dr. Vadim Jucaud’s Lab is recognized for its vibrant and collaborative research environment, where multidisciplinary teams thrive and innovation flourishes. With access to state-of-the-art facilities and cutting-edge equipment, scientists in the lab are empowered to push the boundaries of scientific discovery in four major research thematic areas:
(1) Organ on-a-chip technologies
(2) Organ transplantation
(3) Biosensors and biomarker detection
(4) Biomaterials
These research thematics seamlessly overlap, thus fostering a holistic approach to advancing biomedical innovation. Dr. Jucaud has authored 77 peer-reviewed publications (52 original research papers and 25 review papers) and 48 conference abstracts, with an h-index of 28 and over 2,355 citations.
Citations (h-index: 27)
SHAPING THE FUTURE OF ORGANS-ON-A-CHIP
We are reimagining what’s possible with Organ-on-a-Chip (OoC) technologies, making them more accessible, scalable, and clinically impactful. Our mission is to break down barriers by reducing fabrication costs and eliminating the need for expensive infrastructure, accelerating the path from lab discovery to real-world patient care. Our research drives innovation across several critical fields:
(1) Organ Transplantation: Our liver-on-a-chip platform enhances organ preservation and reduces rejection risks, improving transplant outcomes and saving more lives.
(2) Cancer Immunotherapy: Our lymph node-on-a-chip model enables the development of personalized cancer vaccines by simulating the body’s immune response to tumors.
(3) Neurological Disease: Our blood-brain barrier-on-a-chip opens new possibilities for targeted drug delivery to the brain, advancing treatments for Alzheimer’s, Parkinson’s, and more.
(4) Brain Cancer Research: Our glioblastoma-on-a-chip model recreates the tumor microenvironment, enabling faster, more effective drug discovery against aggressive brain cancers.
ADVANCING TRANSPLANT IMMUNOLOGY
We are dedicated to uncovering how donor-specific HLA antibodies shape outcomes in organ transplantation. By mapping how antibodies interact with specific HLA molecules and developing predictive models of immune responses, we aim to personalize transplant strategies and improve patient outcomes. Our research focuses on three key goals:
(1) Defining the prevalence, incidence, and clinical impact of HLA antibodies.
(2) Characterizing antibody-specific epitopes that drive immune recognition.
(3) Predicting HLA immunogenicity to better match donors and recipients.
Our work also explores pathways to transplant tolerance, finding ways to achieve long-term graft acceptance without lifelong immunosuppression. Through these efforts, we are building a foundation for safer, more effective, and more personalized transplantation therapies.
BIOSENSORS AND BIOMARKER DETECTION
We develop advanced biosensors to monitor biological systems in real time. Integrated with organ-on-a-chip platforms, these sensors enable dynamic tracking of cell behavior, drug responses, and biomarker secretion in physiologically relevant environments. Our technologies include:
(1) Label-free electrochemical and optical sensors
(2) TEER (trans-epithelial electrical resistance) sensors
(3) Solid-phase immunoassays (Luminex, ELISA)
(4) Micro cell-based ELISA systems
We are also advancing non-invasive approaches, such as contact lens biosensors for monitoring ocular biomarkers. By combining biosensing and organ-on-a-chip technologies, we aim to accelerate disease research, drug discovery, and personalized medicine.
BIOMATERIALS
We explore how biomaterials, biosensors, and organ-on-a-chip technologies can work together to accelerate clinical translation. By integrating biomaterials into microphysiological systems, we create models that more accurately replicate human organ complexity. This approach enhances biomaterial evaluation in realistic environments, driving advancements in drug screening, disease modeling, and personalized medicine. Through these synergistic innovations, we aim to bridge the gap between research and clinical care, ultimately improving patient outcomes.
ALUMNI
RESEARCH PAPERS
52. Salih ARC, Peirsman A, Khorsandi D, Ferrao R, Ferreira L, Kamaraj M, John JV, Baquerizo A, Jucaud V. Liver Tissueoid on-a-Chip Modeling Liver Regeneration and Allograft Rejection. Advanced Materials. 2026. e21178. doi: 10.1002/adma.202521178.
51. Tang RC, Palanisamy C, Ghosh R, Lee M, Han GR, Nguyen MC, Shen X, Jucaud V, Ozcan A, Di Carlo D. Rapid Point-of-Care Inflammatory Cytokine Monitoring during Normothermic Liver Perfusion via a Multiplexed Paper-Based Vertical Flow Assay. ACS Sensors. 2025. Article ASAP. doi: 10.1021/acssensors.5c01902.
50. Maity S, Hassani Najafabadi A, Kawakita S, Khorsandi D, Yilgor C, Jewell C, Mohaghegh M, Dokmeci MR, Khademhosseini A, Jucaud V. Lymph node paracortex-inspired on-a-chip recapitulating immunosenescence: a cancer vaccine immunogenicity and antitumoral efficacy screening platform. Lab on a Chip. 2025. doi: 10.1039/D5LC00533G.
49. Hernandez AL, Mandal K, Santamaria B, Quintero S, Dokmeci MR, Jucaud V, Holgado M. Continuous Optical Biosensing of IL-8 Cancer Biomarker Using a Multimodal Platform. Bioengineering. 2025; 12(10):1115. doi: 10.3390/bioengineering12101115.
48. Khorsandi D, Yang JW, Trabucco L, Ferrao R, Ferreira L, Dokmeci MR, Khademhosseini A, Ye JY, Jucaud V. Real-Time and Label-Free Monitoring of Monoclonal Antibody Secretion Rates Using a PC-TIR Biosensor. Biosensors and Bioelectronics. 2025; 117979. doi: 10.1016/j.bios.2025.117979.
47. Nguyen HT, Tirpáková Z, Peirsman A, Maity S, Falcone N, Kawakita S, Jeon K, Khorsandi D, Rashad A, Farhadi N, Mandal K, Ermis M, Herculano R, Hassani Najafabadi A, Dokmeci MR, de Barros NR, Khademhosseini A, Jucaud V. Embolization-on-a-chip: Novel Vascularized Liver Tumor Model for Evaluation of Cellular and Cytokine Response to Embolic Agents. Biofabrication. 2025; In Press. doi: 10.1088/1758-5090/adfbc3.
46. Maity S, Jewell C, Yilgor C, Kawakita S, Sharma S, Gomez A, Mecwan M, Falcone N, Ermis M, Monirizad M, Kouchehbaghi NH, Zehtabi F, Khorsandi D, Dokmeci MR, Moniz-Garcia D, Quiñones-Hinojosa A, Khademhosseini A, Jucaud V. Deciphering pericyte-induced temozolomide resistance in glioblastoma with a 3D microphysiological system mimicking the biomechanical properties of brain tissue. Acta Biomaterialia. 2025; In Press. doi: 10.1016/j.actbio.2025.05.038.
45. Maity S, Hassani Najafabadi A, Kawakita S, Khorsandi D, Yilgor C, Jewell C, Mohaghegh M, Dokmeci MR, Khademhosseini A, Jucaud V. Modeling Immunosenescence on-a-chip: a platform for cancer vaccine efficacy assessment. bioRxiv. 2025; 05.14.654059. doi: 10.1101/2025.05.14.654059.
44. Nguyen HT, Tirpáková Z, Peirsman A, Maity S, Falcone N, Kawakita S, Khorsandi D, Rashad A, Farhadi N, Mandal K, Ermis M, Herculano R, Hassani Najafabadi A, Dokmeci MR, de Barros NR, Khademhosseini A, Jucaud V. Embolization-on-a-chip: Novel Vascularized Liver Tumor Model for Evaluation of Cellular and Cytokine Response to Embolic Agents. bioRxiv. 2025; 03.30.646225. doi: 10.1101/2025.03.30.646225.
43. Joshi A, Kamaraj M, Moghimi N, Heidari H, Aghamaleky-Sarvestany A, Salih ARC, Rodriguez-Sanchez DN, Hu C, Aravindan S, Nair DSR, Huang NF, Thomas BB, Sareen D, Yoshihara E, Jucaud V, Khademhosseini A, John JV. Filamented Light (FLight) Biofabrication of Aligned Fibrillar Structures to Direct 3D Cell Organization Within Microgels. Small. 2025; 2500261. doi: 10.1002/smll.202500261.
42. de Barros NR, Chagas ALD, Forster S, Sant'Ana Pegorin Brasil G, Ribeiro GM, Olivato OP, Ramalho LNZ, Guerra NB, Mussagy CU, Floriano JF, Barbosa AMP, Rudge MVC, Silva RG, Farhadi N, Mohaghegh N, Monirizad M, Peirsman A, Gomez A, de Mendonça RJ, dos Santos LS, Marcaccini AM, Tognoli S, Binotto CCS, Domingues AN, Jucaud V, Mendes AA, Oliveira AS, Li B, Herculano RD. Novel highly flexible collagen-loaded latex dressing to treat diabetic foot ulcer: A pilot clinical trial. International Journal of Biological Macromolecules. 2025; 308: 142615. doi: 10.1016/j.ijbiomac.2025.142615.
41. Mathes TG, Kim U, Jeon K, Estevez PJ, Terasaki M, Ermis M, O'Raw A, Jucaud V, Khademhosseini A, Falcone N. Lipopeptide Hydrogel Possesses Adjuvant-Like Properties for the Delivery of the GPC-3 Peptide-derived Antigen. Adv. Funct. Mater. 2025, 2413870. doi: 10.1002/adfm.202413870.
40. Kamaraj M, Moghimi N, McCarthy A, Chen J, Cao S, Salih ARC, Joshi A, Jucaud V, Panayi A, Shin SR, Noshadi I, Khademhosseini A, Xie J, John JV. Granular Porous Nanofibrous Microspheres Enhance Cellular Infiltration for Diabetic Wound Healing. ACS Nano. 2024; Article ASAP. doi: 10.1021/acsnano.4c10044.
39. Mohaghegh N, Ahari A, Buttles C, Davani S, Hoang H, Huang Q, Huang Y, Hosseinpour B, Abbasgholizadeh R, Cottingham AL, Farhadi N, Akbari M, Kang H, Khademhosseini A, Jucaud V, Pearson RM, and Hassani Najafabadi A. Simvastatin-Loaded Polymeric Nanoparticles: Targeting Inflammatory Macrophages for Local Adipose Tissue Browning in Obesity Treatment. ACS Nano. 2024; Article ASAP. doi: 10.1021/acsnano.4c10742.
38. Yang JW, Khorsandi D, Trabucco L, Ahmed M, Khademhosseini A, Dokmeci MR, Ye JY, Jucaud V. Liver-on-a-Chip Integrated with Label-Free Optical Biosensors for Rapid and Continuous Monitoring of Drug-Induced Toxicity. Small. 2024; 2403560. doi: 10.1002/smll.202403560.
37. Maity S, Jewell C, Yilgor C, Kawakita S, Sharma S, Gomez A, Mecwan M, Falcone N, Ermis M, Monirizad M, Kouchehbaghi NH, Zehtabi F, Khorsandi D, Dokmeci MR, Moniz-Garcia D, Quiñones-Hinojosa A, Khademhosseini A, Jucaud V. Microphysiological system modeling pericyte-induced temozolomide resistance in glioblastoma. bioRxiv. 2024; 07.16.603611. doi: 10.1101/2024.07.16.603611.
36. Costabeber G, Guerra NB, Brasil GSAP, Sasaki JCdS, Scontri M, Burd BS, Su Y, Tanaka JL, Mandal K, Mecwan M, Farhadi N, Gómez A, Ma C, Mussagy CU, Silva GR, dos Santos LS, de Barros NR, Barbosa GF, Jucaud V, Li B, Herculano RD. Additive manufacturing of polylactic acid scaffolds dip-coated with polycaprolactone for bone tissue engineering. Materials Today Communications. 2024; 40:109646. doi: 10.1016/j.mtcomm.2024.109646.35.
35. Mathes TG, Monirizad M, Ermis M, de Barros NR, Rodriguez M, Kraatz HB, Jucaud V, Khademhosseini A, Falcone N. Effects of amyloid-β-mimicking peptide hydrogel matrix on neuronal progenitor cell phenotype. Acta Biomaterialia. 2024. doi: 10.1016/j.actbio.2024.05.020.
34. Rezaei Z, Navarro Torres A, Ge D, Wang T, Méndez Terán EC, García Vera SE, Bassous NJ, Soria OYP, Ávila Ramírez AE, Flores Campos LM, Azuela Rosas DA, Hassan S, Khorsandi D, Jucaud V, Hussain MA, Khateeb A, Zhang YS, Lee H, Kim DH, Khademhosseini A, Dokmeci MR, Shin SR. Noninvasive and Continuous Monitoring of On-Chip Stem Cell Osteogenesis Using a Reusable Electrochemical Immunobiosensor. ACS Sens. 2024; Apr 19. doi: 10.1021/acssensors.3c02165.
33. Jiang C, Chao CC, Li J, Ge X, Jucaud V, Cheng C, Shen X. A Role of Tissue-Resident Memory T Cells on Melanoma Prognosis: Insights from Singlecell RNA Sequencing Data. iScience. 2024; 27(3):109277. doi: 10.1016/j.isci.2024.109277.
32. Herculano RD, Dos Reis CE, de Souza SMB, Pegorin Brasil GS, Scontri M, Kawakita S, Carvalho BG, Bebber CC, Su Y, de Sousa Abreu AP, Mecwan MM, Mandal K, Fusco Almeida AM, Mendes Giannini MJS, Guerra NB, Mussagy CU, Bosculo MRM, Gemeinder JLP, de Almeida BFM, Floriano JF, Farhadi N, Monirizad M, Khorsandi D, Nguyen HT, Gomez A, Tirpáková Z, Peirsman A, da Silva Sasaki JC, He S, Forster S, Burd BS, Dokmeci MR, Terra-Garcia M, Junqueira JC, de Mendonça RJ, Cardoso MR, Dos Santos LS, Silva GR, Barros NR, Jucaud V, Li B. Amphotericin B-loaded natural latex dressing for treating Candida albicans wound infections using Galleria mellonella model. J Control Release. 2023; 13;365:744-758. doi: 10.1016/j.jconrel.2023.12.010.
31. Kawakita S, Li S, Nguyen HT, Maity S, Haghniaz R, Bahari J, Yu N, Mandal K, Bandaru P, Mou L, Ermis M, Khalil E, Khosravi S, Peirsman A, Nasiri R, Adachi A, Nakayama A, Bell R, Zhu Y, Jucaud V, Dokmeci MR, Khademhosseini A. Rapid integration of screen-printed electrodes into thermoplastic organ-on-a-chip devices for real-time monitoring of trans-endothelial electrical resistance. Biomedical Microdevices. 2023; 25:37. doi: 10.1007/s10544-023-00669-9.
30. Abdalla G, Mussagy CU, Sant'Ana Pegorin Brasil G, Scontri M, da Silva Sasaki JC, Su Y, Bebber C, Rocha RR, de Sousa Abreu AP, Goncalves RP, Burd BS, Pacheco MF, Romeira KM, Picheli FP, Guerra NB, Farhadi N, Floriano JF, Forster S, He S, Nguyen HT, Peirsman A, Tirpáková Z, Huang S, Dokmeci MR, Ferreira ES, dos Santos LS, Piazza RD, Marques RFC, Goméz A, Jucaud V, Li B, de Azeredo HMC, Herculano RD. Eco-sustainable coatings based on chitosan, pectin, and lemon essential oil nanoemulsion and their effect on strawberry preservation. International Journal of Biological Macromolecules. 2023; 249:126016. doi: 10.1016/j.ijbiomac.2023.126016.
29. Herculano RD, Dos Santos TO, de Barros NR, Pegorin Brasil GS, Scontri M, Carvalho BG, Mecwan M, Farhadi N, Kawakita S, Perego CH, Carvalho FA, Dos Santos AG, Guerra NB, Floriano JF, Mussagy CU, Tirpáková Z, Khorsandi D, Peirsman A, Nguyen HT, Gomez A, Mandal K, de Mendonça RJ, Li B, Dokmeci MR, Jucaud V. Aloe vera-loaded natural rubber latex dressing as a potential complementary treatment for psoriasis. Int J Biol Macromol. 2023; 11;242(Pt 1):124779. doi: 10.1016/j.ijbiomac.2023.124779.
28. de Souza Silva FK, Orlandi CBC, Fernandes MA, Sant'Ana Pegorin Brasil G, Mussagy CU, Scontri M, Sasaki JC, de Sousa Abreu AP, Guerra NB, Floriano JF, de Mendonça RJ, Caetano GF, Farhadi N, Gómez A, Huang S, Farias AM, Primo FL, Li B, Almeida AMF, Dokmeci MR, Jucaud V, Giannini MJSM, Cardoso MR, Herculano RD. Biocompatible anti-aging face mask prepared with curcumin and natural rubber with antioxidant properties. Int J Biol Macromol. 2023; 10:124778. doi: 10.1016/j.ijbiomac.2023.124778.
27. Wilson N, Reese S, Ptak L, Aziz F, Parajuli S, Jucaud V, Denham S, Mishra A, Cascalho M, Platt JL, Hematti P, Djamali A. Ixazomib for Desensitization (IXADES) in Highly Sensitized Kidney Transplant Candidates: A Phase II Clinical Trial. Kidney360. 2023; Mar 23. doi: 10.34067/KID.0000000000000113.
26. Mandal K, Sangabathuni S, Haghniaz R, Mecwan M, Kawakita S, Nakayama A, Zhang C, Edalati E, Huang W, Hernandez AL, Jucaud V, Dokmeci MR, Khademhosseini A. Oxygen-generating microparticles downregulate HIF-1α expression, increase cardiac contractility, and mitigate ischemic injury. Acta Biomaterialia. 2023; 159: 211-225. doi: 10.1016/j.actbio.2023.01.030.
25. Ge G, Mandal K, Haghniaz R, Li M, Xiao X, Carlson L, Jucaud V, Dokmeci MR, Ho GW, Khademhosseini A. Deep Eutectic Solvents-Based Ionogels with Ultrafast Gelation and High Adhesion in Harsh Environments. Adv. Funct. Mater. 2023; 33(9): 2207388. doi: 10.1002/adfm.202207388.
24. Mecwan MM, Haghniaz R, Hassani A, Mandal K, Jucaud V, John JV, and Khademhosseini A. Thermoresponsive shear-thinning hydrogel (T-STH) hemostats for minimally invasive treatment of external hemorrhages. Biomater Sci. 2023; 31;11(3):949-963. doi: 10.1039/d2bm01559e.
23. Zhu Y, Nasiri R, Davoodi E, Zhang S, Saha S, Linn M, Jiang L, Haghniaz R, Hartel MC, Jucaud V, Dokmeci MR, Herland A, Toyserkani E, Khademhosseini A. A Microfluidic Contact Lens to Address Contact Lens-Induced Dry Eye. Small. 2022; 2207017. doi:10.1002/smll.202207017.
22. de Paiva MB, Pegorin Brasil GS, Destro Chagas AL, Macedo AP, Ramos J, Mardegan Issa JP, Gangrade A, Ferreira Floriano J, Ferreira Caetano G, Li B, Farhadi N, Mandal K, Dokmeci MR, Jucaud V, Herculano RD, and Shimano AC. Latex-collagen membrane: an alternative treatment for tibial bone defects. J Mater Sci. 2022. doi: 10.1007/s10853-022-08009-7.
21. Li S, Zhu Y, Haghniaz R, Kawakita S, Guan S, Chen J, Li Z, Mandal K, Bahari J, Shah S, Guo J, Kang H, Sun W, Kim H-J, Jucaud V, Dokmeci MR, Kollbaum P, Lee CH, Khademhosseini A. A Microchambers Containing Contact Lens for the Noninvasive Detection of Tear Exosomes. Adv Funct Mater. 2022: 2206620. doi: 10.1002/adfm.202206620.
20. Zhu Y, Hartel MC, Yu N, Garrido PR, Kim S, Lee J, Bandaru P, Guan S, Lin H, Emaminejad S, de Barros NR, Ahadian S, Kim H-J, Sun W, Jucaud V, Dokmeci MR, Weiss PS, Yan R, Khademhosseini A. Epidermis-Inspired Wearable Piezoresistive Pressure Sensors Using Reduced Graphene Oxide Self-Wrapped Copper Nanowire Networks. Small Methods. 2022; 6(1):2100900. doi: 10.1002/smtd.202100900.
19. Lee J, Wang Y, Xue C, Chen Y, Qu M, Thakor J, Zhou X, Barros NR, Falcone N, Young P, van den Dolder FW, Lee K, Zhu Y, Cho H-J, Sun W, Zhao B, Ahadian S, Jucaud V, Dokmeci MR, Khademhosseini A, Kim H-J. pH-Responsive doxorubicin delivery using shear-thinning biomaterials for localized melanoma treatment. Nanoscale. 2022; 14(2):350-60. doi: 10.1039/D1NR05738C.
18. Zhu Y, Kim S, Ma X, Byrley P, Yu N, Liu Q, Sun X, Xu D, Peng S, Hartel MC, Zhang S, Jucaud V, Dokmeci MR, Khademhosseini A, Yan R. Ultrathin-shell epitaxial Ag@Au core-shell nanowires for high-performance and chemically-stable electronic, optical, and mechanical devices. Nano Research. 2021; 14(11):4294-303. doi: 10.1007/s12274-021-3718-z.
17. Kawakita S, Beaumont JL, Jucaud V, Everly MJ. Personalized prediction of delayed graft function for recipients of deceased donor kidney transplants with machine learning. Scientific Reports. 2020; 10(1):18409. doi: 10.1038/s41598-020-75473-z.
16. Jucaud V, Nguyen A, Tran B, Hopfield J, Pham T. Validation and cross-reactivity pattern assessment of monoclonal antibodies used for the screening of donor-specific IgG antibody subclasses in transplant recipients. J Immunol Methods. 2020; 486: 112847. doi: 10.1016/j.jim.2020.112847.
15. Chen Y, Zhang S, Cui Q, Ni J, Wang X, Cheng X, Alem H, Tebon P, Xu C, Guo C, Nasiri R, Moreddu R, Yetisen AK, Ahadian S, Ashammakhi N, Emaminejad S, Jucaud V, Dokmeci MR, Khademhosseini A. Microengineered poly(HEMA) hydrogels for wearable contact lens biosensing. Lab on a Chip. 2020; 20(22):4205-14. doi: 10.1039/D0LC00446D.
14. Jucaud V, Shaked A, DesMarais M, Sayre P, Feng S, Levitsky J, Everly MJ. Prevalence and Impact of De Novo Donor-Specific Antibodies During a Multicenter Immunosuppression Withdrawal Trial in Adult Liver Transplant Recipients. Hepatology. 2019; 69(3):1273-86. doi: 10.1002/hep.30281.
13. Ravindranath MH, Jucaud V, Ferrone S. Monitoring native HLA-I trimer specific antibodies in Luminex multiplex single antigen bead assay: Evaluation of beadsets from different manufacturers. J Immunol Methods. 2017; 450:73-80. doi: 10.1016/j.jim.2017.07.016.
12. Ravindranath MH, Jucaud V, Banuelos N, Everly MJ, Cai J, Nguyen A, Terasaki PI. Nature and Clonality of the Fluoresceinated Secondary Antibody in Luminex Multiplex Bead Assays Are Critical Factors for Reliable Monitoring of Serum HLA Antibody Levels in Patients for Donor Organ Selection, Desensitization Therapy, and Assessment of the Risk for Graft Loss. J Immunol. 2017; 198(11):4524-38. doi: 10.4049/jimmunol.1700050.
11. Jucaud V, Ravindranath MH, Terasaki PI. Conformational Variants of the Individual HLA-I Antigens on Luminex Single Antigen Beads Used in Monitoring HLA Antibodies: Problems and Solutions. Transplantation. 2017; 101(4):764-77. doi: 10.1097/TP.0000000000001420.
10. Jucaud V. The Immunogenicity of HLA Class II Mismatches: The Predicted Presentation of Nonself Allo-HLA-Derived Peptide by the HLA-DR Phenotype of the Recipient Is Associated with the Formation of DSA. J Immunol Res. 2017; 2017:2748614. doi: 10.1155/2017/2748614.
9. Hilali FE, Jucaud V, Hilali HE, Bhuiyan MH, Mancuso A, LiuSullivan N, Elidrissi A, Mazouz H. Characterization of the Anti-HLA Class I and II IgG Antibodies in Moroccan IVIg Using Regular Beads and Ibeads in Luminex Multiplex Single Antigen Immunoassay. International Journal of Immunology. 2017; 5(4):53-65. doi: 10.11648/j.iji.20170504.11.
8. Ravindranath MH, Jucaud V, Maehara CY, Terasaki PI. Significance of the differences in the prevalence of anti-HLA antibodies in matched pairs of mother’s and cord blood. Immunology Letters. 2016; 170:68-79. doi: 10.1016/j.imlet.2015.11.016.
7. Jucaud V, Ravindranath MH, Terasaki PI, Morales-Buenrostro LE, Hiepe F, Rose T, Biesen R. Serum antibodies to human leucocyte antigen (HLA)-E, HLA-F and HLA-G in patients with systemic lupus erythematosus (SLE) during disease flares: Clinical relevance of HLA-F autoantibodies. Clin Exp Immunol. 2016; 183(3):326-40. doi: 10.1111/cei.12724.
6. Ravindranath MH, Terasaki PI, Pham T, Jucaud V. The Monospecificity of Novel Anti-HLA-E Monoclonal Antibodies Enables Reliable Immunodiagnosis, Immunomodulation of HLA-E, and Upregulation of CD8+ T Lymphocytes. Monoclon Antib Immunodiagn Immunother. 2015; 34(3):135-53. doi: 10.1089/mab.2014.0096.
5. Ravindranath MH, Terasaki PI, Maehara CY, Jucaud V, Kawakita S, Pham T, Yamashita W. Immunoglobulin (Ig)G purified from human sera mirrors intravenous Ig human leucocyte antigen (HLA) reactivity and recognizes one's own HLA types, but may be masked by Fab complementarity-determining region peptide in the native sera. Clin Exp Immunol. 2015; 179(2):309-28. doi: 10.1111/cei.12450.
4. Zhu D, Ravindranath MH, Terasaki PI, Miyazaki T, Pham T, Jucaud V. Suppression of allo-human leucocyte antigen (HLA) antibodies secreted by B memory cells in vitro: intravenous immunoglobulin (IVIg) versus a monoclonal anti-HLA-E IgG that mimics HLA-I reactivities of IVIg. Clin Exp Immunol. 2014; 177(2):464-77. doi: 10.1111/cei.12307.
3. Sasaki T, Ravindranath MH, Terasaki PI, Freitas MC, Kawakita S, Jucaud V. Gastric cancer progression may involve a shift in HLA-E profile from an intact heterodimer to β2-microglobulin-free monomer. International Journal of Cancer. 2014; 134(7):1558-70. doi: 10.1002/ijc.28484.
2. Ravindranath MH, Terasaki PI, Pham T, Jucaud V, Kawakita S. Suppression of blastogenesis and proliferation of activated CD4(+) T cells: intravenous immunoglobulin (IVIg) versus novel anti-human leucocyte antigen (HLA)-E monoclonal antibodies mimicking HLA-I reactivity of IVIg. Clin Exp Immunol. 2014; 178(1):154-77. doi: 10.1111/cei.12391.
1. Ravindranath MH, Terasaki PI, Pham T, Jucaud V, Kawakita S. Therapeutic preparations of IVIg contain naturally occurring anti–HLA-E antibodies that react with HLA-Ia (HLA-A/-B/-Cw) alleles. Blood. 2013; 121(11):2013-28. doi: 10.1182/blood-2012-08-447771.
REVIEW PAPERS
25. Mohaghegh N, Balajam NZ, Hosseinpour B, Buttles C, Huang Q, Huang Y, Ahari A, Farhadi N, Davani S, Khosravi S, Mirmashhouri B, Kouchehbaghi NH, Sampath R, Akbari M, Jucaud V, Kang H, Khademhosseini A, Pearson RM, Hassani Najafabadi A. Immunoengineering strategies using nanoparticles for obesity treatment. Nano Research. 2025. doi: 10.26599/NR.2025.94907707.
24. Burd BS, Mussagy CU, Bebber C, Brasil GSP, dos Santos LS, Guerra NB, Persinoti GF, Jucaud V, Goldbeck R, Herculano RD. Can the insects Galleria mellonella and Tenebrio molitor be the future of plastic biodegradation? Science of The Total Environment. 2025; 969: 178879. doi: 10.1016/j.scitotenv.2025.178879.
23. Maity S, Bhuyan T, Jewell C, Kawakita S, Sharma S, Nguyen HT, Hassani Najafabadi A, Ermis M, Falcone N, Chen J, Mandal K, Khorsandi D, Yilgor C, Choroomi A, Torres E, Mecwan M, John JV, Akbari M, Wang Z, Moniz-Garcia D, Quiñones-Hinojosa A, Jucaud V, Dokmeci MR, Khademhosseini A. Recent Developments in Glioblastoma-On-A-Chip for Advanced Drug Screening Applications. Small. 2024; e2405511. doi: 10.1002/smll.202405511.
22. Khorsandi D, Yang J, Jenson S, Kajino T, Maity S, Salih ARC, Jucaud V, Dokmeci MR. Lab-on-a-chip: Unit Operations to Scale-up Strategies. In: Lab-on-a-chip Devices for Advanced Biomedicines: Laboratory Scale Engineering to Clinical Ecosystem. Royal Society of Chemistry. Ed: Parihar A, Pradeep Mehta P. 2024: 25 (17): 560-614.21. doi: 10.1039/9781837673476
21. Jucaud V. Allogeneic HLA Humoral Immunogenicity and the Prediction of Donor-Specific HLA Antibody Development. Antibodies. 2024, 13(3), 61. doi: 10.3390/antib13030061.
20. Marques PAC, Guerra NB, Dos Santos LS, Mussagy CU, Sant'Ana Pegorin Brasil G, Burd BS, Su Y, da Silva Sasaki JC, Scontri M, de Lima Lopes Filho PE, Silva GR, Miranda MCR, Ferreira ES, Primo FL, Fernandes MA, Crotti AEM, He S, Forster S, Ma C, de Barros NR, de Mendonça RJ, Jucaud V, Li B, Herculano RD, Floriano JF. Natural rubber latex-based biomaterials for drug delivery and regenerative medicine: Trends and directions. Int J Biol Macromol. 2024;16:131666. doi: 10.1016/j.ijbiomac.2024.131666.
19. Khorsandi D, Yang JW, Foster S, Khosravi S, Hosseinzadeh Kouchehbaghi N, Zarei F, Lee YB, Runa F, Gangrade A, Voskanian L, Adnan D, Zhu Y, Wang Z, Jucaud V, Dokmeci MR, Shen X, Bishehsari F, Kelber JA, Khademhosseini A, de Barros NR. Patient-Derived Organoids as Therapy Screening Platforms in Cancer Patients. Adv. Healthcare Mater. 2024; e2302331. doi: 10.1002/adhm.202302331.
18. Zare EN, Khorsandi D, Zarepour A, Yilmaz H, Agarwal T, Hooshmand S, Mohammadinejad R, Ozdemir F, Sahin O, Adiguzel S, Khan H, Zarrabi A, Sharifi E, Kumar A, Mostafavi E, Hosseinzadeh Kouchehbaghi N, Mattoli V, Zhang F, Jucaud V, Hassani Najafabadi A, Khademhosseini A. Biomedical applications of engineered heparin-based materials. Bioactive Materials. 2024; 31:87-118. doi: 10.1016/j.bioactmat.2023.08.002.
17. Mohaghegh N, Ahari A, Zehtabi F, Buttles C, Davani S, Hoang H, Tseng K, Zamanian B, Khosravi S, Daniali A, Kouchehbaghi NH, Thomas I, Nouri H, D Khorsandi, Abasgholizadeh R, Akbari M, Patil R, Kang H, Jucaud V, Khademhosseini A, and Hassani Najafabadi A. Injectable Hydrogels for Personalized Cancer Immunotherapies. Acta Biomaterialia. 2023;172:67-91. doi: 10.1016/j.actbio.2023.10.002.
16. Falcone N, Ermis M, Tamay DG, Mecwan M, Monirizad M, Mathes TG, Jucaud V, Choroomi A, Barros N, Zhu Y, Vrana NE, Kraatz HB, Kim HJ, Khademhosseini A. Peptide Hydrogels as Immunomaterials and Their Use in Cancer Immunotherapy Delivery. Adv. Healthcare Mater. 2023; 2301096. doi: 10.1002/adhm.202301096.
15. Karamikamkar S, Yalcintas EP, Haghniaz R, de Barros NR, Mecwan M, Nasiri R, Davoodi E, Nasrollahi F, Erdem A, Kang H, Lee J, Zhu Y, Ahadian S, Jucaud V, Maleki H, Dokmeci MR, Kim HJ, Khademhosseini A. Aerogel-Based Biomaterials for Biomedical Applications: From Fabrication Methods to Disease-Targeting Applications. Adv. Sci. 2023; 2204681. doi: 10.1002/advs.202204681.
14. Peirsman A, Nguyen HT, Van Waeyenberge M, Ceballos-González C, Bolívar-Monsalve J, Kawakita S, Vanlauwe F, Tirpáková Z, Van Dorpe S, Van Damme L, Mecwan M, Ermis M, Maity S, Mandal K, Herculano R, Depypere B, Budiharto L, Van Vlierberghe S, De Wever O, Blondeel P, Jucaud V, Dokmeci MR, Khademhosseini A. Vascularized adipose tissue engineering: moving towards soft tissue reconstruction. Biofabrication. 2023; 15(3). doi: 10.1088/1758-5090/acd7a5.
13. Nguyen HT, Peirsman A, Tirpakova Z, Mandal K, Vanlauwe F, Maity S, Kawakita S, Khorsandi D, Herculano R, Umemura C, Yilgor C, Bell R, Hanson A, Li S, Nanda HS, Zhu Y, Najafabadi AH, Jucaud V, Barros N, Dokmeci MR, Khademhosseini A. Engineered Vasculature for Cancer Research and Regenerative Medicine. Micromachines. 2023; 14(5):978. doi: 10.3390/mi14050978.
12. Mou L, Mandal K, Mecwan MM, Hernandez AL, Maity S, Sharma S, Herculano RD, Kawakita S, Jucaud V, Dokmeci MR, Khademhosseini A. Integrated biosensors for monitoring microphysiological systems. Lab on a Chip. 2022. 24(8):2358-2359. doi: 10.1039/D2LC00262K.
11. Kawakita S, Mandal K, Mou L, Mecwan MM, Zhu Y, Li S, Sharma S, Hernandez AL, Nguyen HT, Maity S, de Barros NR, Nakayama A, Bandaru P, Ahadian S, Kim H-J, Herculano RD, Holler E, Jucaud V, Dokmeci MR, Khademhosseini A. Organ-On-A-Chip Models of the Blood–Brain Barrier: Recent Advances and Future Prospects. Small. 2022; 18(39):e2201401. doi: 10.1002/smll.202201401.
10. Zhu Y, Li S, Li J, Falcone N, Cui Q, Shah S, Hartel MC, Yu N, Young P, de Barros NR, Wu Z, Haghniaz R, Ermis M, Wang C, Kang H, Lee J, Karamikamkar S, Ahadian S, Jucaud V, Dokmeci MR, Kim H-J, Khademhosseini A. Lab-on-a-Contact Lens: Recent Advances and Future Opportunities in Diagnostics and Therapeutics. Advanced Materials. 2022; 34(24):e2108389. doi: 10.1002/adma.202108389.
9. Zhu Y, Mandal K, Hernandez AL, Kawakita S, Huang W, Bandaru P, Ahadian S, Kim H-J, Jucaud V, Dokmeci MR, Khademhosseini A. State of the art in integrated biosensors for organ-on-a-chip applications. Current Opinion in Biomedical Engineering. 2021;19:100309. doi: 10.1016/j.cobme.2021.100309.
8. Zhu Y, Haghniaz R, Hartel MC, Mou L, Tian X, Garrido PR, Wu Z, Hao T, Guan S, Ahadian S, Kim H-J, Jucaud V, Dokmeci MR, Khademhosseini A. Recent Advances in Bioinspired Hydrogels: Materials, Devices, and Biosignal Computing. ACS Biomaterials Science & Engineering. 2023; 9(5):2048-69. doi: 10.1021/acsbiomaterials.1c00741.
7. Nasrollahi F, Haghniaz R, Hosseini V, Davoodi E, Mahmoodi M, Karamikamkar S, Darabi MA, Zhu Y, Lee J, Diltemiz SE, Montazerian H, Sangabathuni S, Tavafoghi M, Jucaud V, Sun W, Kim H-J, Ahadian S, Khademhosseini A. Micro and Nanoscale Technologies for Diagnosis of Viral Infections. Small. 2021;17(45):2100692. doi: 10.1002/smll.202100692.
6. Ma X, Ahadian S, Liu S, Zhang J, Liu S, Cao T, Lin W, Wu D, de Barros NR, Zare MR, Diltemiz SE, Jucaud V, Zhu Y, Zhang S, Banton E, Gu Y, Nan K, Xu S, Dokmeci MR, Khademhosseini A. Smart Contact Lenses for Biosensing Applications. Advanced Intelligent Systems. 2021;3(5):2000263. doi: 10.1002/aisy.202000263.
5. Zhou X, Jiang X, Qu M, Aninwene GE, Jucaud V, Moon JJ, Gu Z, Sun W, Khademhosseini A. Engineering Antiviral Vaccines. ACS Nano. 2020;14(10):12370-89. doi: 10.1021/acsnano.0c06109.
4. El-Awar N, Jucaud V, Nguyen A. HLA Epitopes: The Targets of Monoclonal and Alloantibodies Defined. J Immunol Res. 2017;2017:3406230. doi: 10.1155/2017/3406230.
3. Ravindranath M, Jucaud V, Terasaki P. Immunobiology of Allograft Human Leukocyte Antigens in the New Microenvironment. SOJ Immunol. 2015;3(4):1-19. doi: 10.15226/2372-0948/3/4/00135.
2. Jucaud V, Ravindranath M, Terasaki P. Immunobiology of HLA class-Ib molecules in transplantation. SOJ Immunol. 2015;3(4):1-15. doi: 10.15226/2372-0948/3/4/00137.
1. Ravindranath MH, Zhu D, Pham T, Jucaud V, Hopfield J, Kawakita S, Terasaki PI. Anti-HLA-E monoclonal antibodies reacting with HLA-la and lb alleles like IVIg as potential IVIg-immunomimetics: an evolving therapeutic concept. Clin Transpl. 2013:293-305. PMID: 25095521.
CONFERENCE ABSTRACTS
48. Salih ARC, Baquerizo A, Jucaud V. Vascularized Liver Tissueoid-on-a-Chip: An In Vitro Model for Studying Allograft Rejection. Am J Transplant. 2025; 25 (8), S60. Oral presentation. World Transplant Congress 2025 (San Fransico, USA).
47. Jiang C, Kawakita S, Jucaud V, Wang J, Elrefaei M, Stegall M, Schinstock C, Jaramillo A, Zhu Y, Hacke K, Roberts J, Khamash H, Khademhosseini A, Taner C, Shen X, Jarmi T. Machine Learning-Based Risk Prediction of Allograft Rejection within One-Year Post-Transplant for Kidney Transplant Patients. Am J Transplant. 2025; 25 (8), S67. Oral presentation. World Transplant Congress 2025 (San Fransico, USA).
46. Baquerizo A, Jucaud V, Keshavarz A, Markmann J. Xenogeneic Profiling of Human Plasma Protein Abundance Following Pig Hepatocyte Exposure during Bioartificial Liver Treatments. Am J Transplant. 2025; 25 (8), S558. Poster presentation. World Transplant Congress 2025 (San Fransico, USA).
45. Hassani Najafabadi A, Mohaghegh N, Khademhosseini A, Pearson RM, Jucaud V, Buttles C. Simvastatin-Loaded Polymeric Nanoparticles: Targeting Inflammatory Macrophages for Local Adipose Tissue Browning in Obesity Treatment. Oral presentation. Biomedical Engineering Society Annual Meeting 2024 (Baltimore, USA).
44. Nguyen HT, Tirpakova Z, Barros N, Falcone N, Mecwan M, Elsebahy A, Ermis M, Khorsandi D, Peirsman A, Herculano R, Hassani-Najafabadi A, Dokmeci MR, Jucaud V, Khademhosseini A. Novel Vascularized Liver Tumor Model For Evaluation Of Vessel Regression And Cell Death Response To Embolic Agents. Oral presentation. Biomedical Engineering Society Annual Meeting 2024 (Baltimore, USA).
43. Falcone N, Mathes T, Jucaud V, Khademhosseini A. Lipopeptide-based hydrogel possesses immune-modulating adjuvant-like properties for the delivery of the GPC-3 peptide-derived antigen. Poster presentation. Biomedical Engineering Society Annual Meeting 2024 (Baltimore, USA).
42. Yang JW, Khorsandi D, Trabucco L, Ahmed M, Khademhosseini A, Ye JY, Dokmeci MR, Jucaud V. Liver-on-a-Chip Integrated with Label-free Optical Biosensors for Real-time Monitoring of Drug-induced Toxicity. Oral presentation. Biomedical Engineering Society Annual Meeting 2024 (Baltimore, USA).
41. Khorsandi D, Yang JW, Khademhosseini A, Ye JY, Dokmeci MR, Jucaud V. Real-time and Label-free Monitoring of Monoclonal Antibody Secretion Rate Using a PC-TIR Sensor. Oral presentation. Biomedical Engineering Society Annual Meeting 2024 (Baltimore, USA).
40. Salih ARC, Moniz D, Khademhosseini A, Quiñones-Hinojosa A, Jucaud V. Patient-Derived Glioblastoma Models for Personalized Medicine: TMZ Chemoresistance Screening. Poster presentation. Biomedical Engineering Society Annual Meeting 2024 (Baltimore, USA).
39. Baquerizo A, Jucaud V. Differential expression of human xenoantibodies to wt and GalT-KO pig endothelial cells after patients exposure to pig hepatocytes following BAL Treatment. Transplantation. 2024; 108 (9S). Oral presentation. International Congress of the Transplantation Society 2024 (Istanbul, Turkey).
38. Kawakita S, Jucaud V, Wang Z, Shen X, Jiang C. Computational immuno-profiling and prediction of antibody-mediated rejection in kidney transplant recipients. Transplantation. 2024; 108 (9S). Poster presentation. International Congress of the Transplantation Society 2024 (Virtual).
37. Jucaud V. Advances in bioengineering research for transplantation. Invited speaker. Expert Talk IMIN 2024 (Virtual).
36. Jucaud V. The Three Pillars of Organs-On-a-Chip Technologies: Where to Innovate? Invited speaker. 11th Annual 3Rs Symposim 2024 (Virtual).
35. Jucaud V. Manufacturing Tomorrow’s Therapeutics: Innovations & Triumphs in Bioprinting. Invited panelist. RAPID + TCT 2024 (Los Angeles, USA).
34. Kawakita S, Jucaud V, Shen X, Jiang C. Transcriptomic signatures of antibody-mediated rejection in kidney transplant recipients. Poster presentation. American Transplant Congress 2024 (Philadelphia, USA).
33. Jucaud V. Brain-on-a-chip Platforms For Drug Discovery, Drug Screening, and Off Target Toxicity Studies. Invited speaker. World congress of the Society For Brain Mapping & Therapeutics 2023 (Los Angeles, USA).
32. Baquerizo A, Jucaud V. Analysis of human anti-porcine immune response in sensitized patients exposed to porcine hepatocytes. Transplantation. 2023; 107 (10S2), S127. Oral presentation. IPITA-IXA-CTRMA 2023 Joint Congress (San Diego, USA).
31. Nguyen HT, Tirpakova Z, Barros N, Falcone N, Mecwan M, Elsebahy A, Ermis M, Khorsandi D, Peirsman A, Herculano R, Hassani-Najafabadi A, Dokmeci MR, Jucaud V, Khademhosseini A. Vascularized liver tumor model for vessel embolic agents testing. Oral presentation. Conference of the European Society for Biomaterials 2023 (Davos, Swizerland).
30. Jucaud V. Recapitulating the Human Body On a Chip: Progress to Date; What’s Missing? Invited speaker. JSNN Nanoimpacts Conference 2022 (Greensboro, USA).
29. Jucaud V. Microscale Sensors and Systems for Tissue Engineering and Regenerative Medicine Applications. Invited speaker. JSNN Nanoimpacts Conference 2022 (Greensboro, USA).
28. Mandal K, Sangabathuni S, Haghniaz R, Mecwan M, Huang W, Kawakita S, Jucaud V, Dokmeci MR, Khademhosseini A. Oxygen-generating microparticles increase cardiac contractility, downregulate HIF-1α expression, and mitigate ischemic injury. Oral presentation. Biomedical Engineering Society Annual Meeting 2022 (San Antonio, USA).
27. Mandal K, Sangabathuni S, Haghniaz R, Mecwan M, Huang W, Jucaud V, Dokmeci MR, Khademhosseini A. Improving cardiomyocyte contractility beating by introducing oxygen releasing microparticles. Oral presentation. Society for biomaterial Annual meeting 2022 (Baltimore, USA).
26. Jucaud V, Rebellato LM, Briley KP, Haisch CE, Kendrick SA, Jones H, McLawhorn K, Leeser D, Everly MJ. Do we predict mismatches that induce de novo DSA or do we predict the ones that do not? Transplantation. 2020; 104 (S3), S49-S50. Oral presentation. International Congress of the Transplantation Society 2020 (Virtual).
25. Jucaud V, Rebellato LM, Briley KP, Haisch CE, Kendrick SA, Jones H, McLawhorn K, Leeser D, Everly MJ. Molecular features of HLA mismatches as predictors of de novo DSA development: a single center experience. Oral presentation. American Transplant Congress 2020 (Virtual).
24. Jucaud V, Rebellato LM, Briley KP, Haisch CE, Kendrick SA, Jones H, McLawhorn K, Leeser D, Everly MJ. HLA mismatch immunogenicity: are we predicating mismatches that induce de novo DSA development or that do not? Poster presentation. American Transplant Congress 2020 (Virtual).
23. Jucaud V, Stegall MD, Schinstock CA, Heilman RL, Rebellato LM, Samaniego-Picota MD, Leeser D, Manish J. Gandhi MJ, Beaumont JL, Everly MJ. Is the Epitope Mismatch Load Enough to Identify HLA-DQ Mismatches with a Low Risk for De Novo DSA Development? Poster presentation. American Transplant Congress 2020 (Virtual).
22. Jucaud V, Babu A, Briggs D, Krishnan N, Mitchell D, Everly MJ. Preformed anti-HLA donor-specific antibodies immunological risk assessment: the importance of HLA antibody locus-specificity. Poster presentation. American Transplant Congress 2020 (Virtual).
21. Jucaud V, Burnett V, Gebel H, Bray R, Polinsky M, Everly MJ. Presenter: Blancho G. Incompatibilités des épitopes et apparition des anticorps spécifiques du donneur (de novo DSA): Une analyse des études BENEFIT et BENEFIT-EXT chez les patients transplantés rénaux. Poster presentation. Societe Francophone de Transplantation 2019 (Bordeaux, France).
20. Jucaud V, Burnett V, Gebel H, Bray R, Polinsky M, Everly MJ. Mismatched epitope and de novo DSA development: An Analysis of the BENEFIT And BENEFIT-EXT Renal Transplant Cohorts. Am J Transplant. 2019; 19:384. Oral presentation. American Transplant Congress 2019 (Boston, USA).
19. Jucaud V, Gebel H, Bray R, Polinsky M, Everly MJ. Impact of HLA-DR matching on HLA-DQ matching status and the development of anti-HLA-DQ de novo DSA under calcineurin inhibitor or belatacept immunosuppression. Am J Transplant. 2019; 19:699-700. Poster presentation. American Transplant Congress 2019 (Boston, USA).
18. Jucaud V, Shaked A, DesMarais M, Sayre P, Feng S, Levitsky J, Everly MJ. Mismatched antibody epitopes and the development of HLA-DQ de novo DSA during immunosuppression withdrawal in liver transplant recipients. Am J Transplant. 2019; 19:1166-167. Poster presentation. American Transplant Congress 2019 (Boston, USA).
17. Babu A, Pham T, Hamdorf M, Jucaud V, Everly MJ, Daga S, Mitchell D. Endothelial Cells Do Not Regulate Expression of CD46, CD55 and CD59 in the Presence of Anti-HLA Class I and II Antibodies. Am J Transplant. 2019; 19:899-900. Poster presentation. American Transplant Congress 2019 (Boston, USA).
16. Babu A, Tran B, Hamdorf M, Jucaud V, Daga S, Mitchell D, Everly MJ. Differential Regulation of Phosphokinases by Anti-HLA Class I and II Antibodies. Am J Transplant. 2019; 19:620. Poster presentation. American Transplant Congress 2019 (Boston, USA).
15. Kawakita S, Rebellato LM, Briley KP, Aldonado AQ, Haisch CE, Bolin P, Kendrick SA, Jones H, McLawhorn K, Leeser D, Jucaud V, Burnett V, Everly MJ. A Prognostic Biomarker to Estimate the likelihood of Developing De Novo Donor Specific Antibody in Kidney Transplant Recipients. Am J Transplant. 2019; 19:638-39. Poster presentation. American Transplant Congress 2019 (Boston, USA).
14. Dadhania D, Park C, Jucaud V, Everly MJ, Friedlander R, Menon AK, Putheti P, Sharma VK, Suthanthiran M. OR17 Epitope load does not predict development of de novo antibodies to HLA in patients with BK virus nephropathy. Hum Immunol. 2018; 79:23. Oral presentation. ASHI 44th Annual Meeting 2018 (Baltimore, USA).
13. Jucaud V, Shaked A, DesMarais M, Sayre P, Everly MJ. Development of post-transplant DSA in adult liver transplant patients from the ITN A-WISH trial. Transplantation. 2018. 102:S203. Oral presentation. International Congress of the Transplantation Society 2018 (Madrid, Spain).
12. Jucaud V, Shaked A, DesMarais M, Sayre P, Everly MJ. The relative immunogenicity of HLA mismatches in adult liver transplant patients from the ITN A-WISH trial. Transplantation. 2018. 102:S623. Poster presentation. International Congress of the Transplantation Society 2018 (Madrid, Spain).
11. Jucaud V, Shaked A, DesMarais M, Sayre P, Everly MJ. Development of post-transplant DSA following immunosuppression minimization/withdrawal in liver transplant recipients. Am J Transplant. 2018; 472-473. Oral presentation. American Transplant Congress 2018 (Seattle, USA).
10. Jucaud V, Roberts M, Gebel H, Bray R, Everly MJ. Determining the relative immunogenicity of HLA mismatches with calcineurin inhibitor immunosuppression: an analysis of the BENEFIT and BENEFIT-EXT renal transplant cohorts. Am J Transplant. 2018; 18:289-290. Oral presentation. American Transplant Congress 2018 (Seattle, USA).
9. Jucaud V, Rebellato LM, Briley KP, Maldonado AQ, Haisch CE, Bolin P, Kendrick SA, Jones H, McLawhorn K, Leeser D, Everly MJ. Not all HLA mismatches are equal: determining the de novo DSA induction capacity of HLA mismatches. Am J Transplant. 2018; 18:443-444. Oral presentation. American Transplant Congress 2018 (Seattle, USA).
8. Everly MJ, Briley KP, Nguyen A, Jucaud V, Maldonado AQ, Haisch CE, Bolin P, Kendrick SA, Jones H, McLawhorn K, Leeser D, Rebellato LM. All DQ DSA May Not Be Equal: Comparing Outcomes between De Novo DQ DSA Specificities. Am J Transplant. 2018; 18:443. Oral presentation. American Transplant Congress 2018 (Seattle, USA).
7. Jucaud V, Shaked A, DesMarais M, Sayre P, Everly MJ. The relative immunogenicity of HLA mismatches in adult liver transplant recipients. Am J Transplant. 2018; 18:626. Poster presentation. American Transplant Congress 2018 (Seattle, USA).
6. Everly MJ, Jucaud V, Roberts M, Gebel H, Bray R. Immunogenic HLA mismatches and evidence that belatacept may be the first therapy able to prevent DQ de novo DSA: an analysis of the BENEFIT and BENEFIT-EXT trial cohorts. Am J Transplant. 2018; 18:780. Poster presentation. American Transplant Congress 2018 (Seattle, USA).
5. Jucaud V, Ravindranath MH, Terasaki PI. Evidence for structural variants within an individual HLA-I molecule coated on Luminex single antigen bead assays: impact on monitoring HLA antibodies for organ allocation and de novo DSA in transplant patients. Transplantation. 2016. 100(7):S377-S378. Oral presentation. International Congress of the Transplantation Society 2016 (Hong Kong, China).
4. Ravindranath MH, Jucaud V, Banuelos N, Nguyen A, Everly MJ, Cai J, Terasaki PI. Importance of the nature and binding specificity of the secondary antibody used in Luminex single antigen bead assays for monitoring clinically relevant HLA IgG antibodies in pre-and post-transplant patients. Transplantation. 2016. 100(7):S375-S376. Oral presentation. International Congress of the Transplantation Society 2016 (Hong Kong, China).
3. Jucaud V, Ravindranath MH, Terasaki PI. Evidence for structural variants within an individual HLA-I molecule coated on Luminex single antigen bead assays: impact on monitoring HLA antibodies for organ allocation and de novo DSA in transplant patients. Am J Transplant. 2016; 16:514-515. Poster presentation. American Transplant Congress 2015 (Boston, USA).
2. Ravindranath MH, Terasaki PI, Maehara C, Jucaud V. Should Current IVIg Preparations Containing High Titers of Anti-HLA-I/-II IgG be Used for Lowering the Anti-HLA IgG in Sensitized Pre-Transplant Patients and Allograft Recipients? Am J Transplant. 2015; 15. Poster presentation. American Transplant Congress 2015 (Philadelphia, USA).
1. Jucaud V, Ravindranath MH, Terasaki PI, Maehara CY, Morales-Buenrostro LE, Biesen R. Antibody levels of HLA-E, HLA-F, HLA-G and beta2-microglobulin (b2m) correlate with disease activity of systemic lupus erythematosus (SLE) based on SLEDAI and BILAG-2004 indices. Poster presentation. International Congress on Autoimmunity 2014 (Nice, France).