DR. NATASHYA FALCONE LABORATORY
ABOUT DR. NATASHYA FALCONE
Dr. Falcone is a Terasaki Fellow at the Terasaki Institute. She received her Ph.D. in May 2021 at the University of Toronto in Chemical Engineering and Applied Chemistry. Here, she worked on synthesizing and characterizing stimuli-responsive peptide-based hydrogels for a variety of applications including enzyme catalysis, cellular support, and drug delivery. She joined the Terasaki Institute in June 2021 and is researching various types of hydrogel biomaterials for the delivery of cancer immunotherapies, for 3D in vitro disease modeling, and in the design of novel immune-modulating materials to increase immunogenic responses of immunotherapies.
Research Overview
Dr. Natashya Falcone's research focuses on the design and development of self-assembling hydrogel biomaterials for a variety of biomedical engineering applications. Dr. Falcone is dedicated to creating bio-inspired materials that can be used as drug delivery systems and that can replicate biological systems, offering new insights into cell-biomaterial interactions and advancing therapeutic and diagnostic applications in immunotherapy, cancer treatment, and regenerative medicine. Key research areas includes:
1. Self-Assembling Hydrogel Biomaterials: We design supramolecular peptide-based, self-assembling hydrogel materials for a variety of applications including drug delivery, ECM-mimicking matrices, vaccine adjuvants, and product formulations.
2. Cancer Immunotherapy: Her current work focuses on developing lipopeptide hydrogels that serve as both adjuvants and delivery vehicles for tumor antigens, enhancing immune responses and improving cancer vaccine efficacy. We also design shear-thinning hydrogels for delivery of immunotherapies.
3. Therapeutic and Disease Modeling: Dr. Falcone has developed innovative hydrogel-based models to study cancer-stroma interactions (e.g., PDAC) and neurodegenerative diseases (e.g., Alzheimer's), providing new platforms for drug testing and disease research.
4. Bio-Inspired Materials for Immune Modulation: By leveraging her expertise in supramolecular assembly, she creates hydrogels that modulate immune responses, offering potential for advancing cancer immunotherapies and regenerative medicine.
Speaking Highlights
Dr. Natashya Falcone was a part of a women in STEM panel at LAVC where she talked about her academic journey and her challenges and advantages being a woman in the STEM field.


Research Highlights
.png)
.png)
In Vitro Cancer Modelling
Crosslinkable hydrogels are used to mimic the extracellular matrices (ECM) of tumors to understand the roles of ECM mechanical properties and components on cancer cell phenotype changes, metastasis, and tumor progression.

Shear-thinning Hydrogels for Drug Delivery
Hydrogels are used for the local delivery of combination therapies, including chemo- and immunotherapies for the treatment of hepatocellular carcinoma, a deadly type of liver cancer.
Peptide-based hydrogel biomaterials
We are designing novel nanofibrous self-assembling peptide-based hydrogels with stimuli-responsive properties, including pH, redox, and thermal responsiveness. These peptide-based hydrogels are specifically designed to support various biomedical applications, such as drug delivery systems, cosmetic formulations, enzyme biocatalysis, and ECM matrix modeling in various diseases. By harnessing the ability of peptides to self-assemble into well-ordered nanostructures, we create multi-functional hydrogel systems capable of responding to environmental cues.

Immune-modulating peptide-based hydrogels
We are currently designing peptide-based hydrogels for the delivery of cancer vaccines while simultaneously possessing adjuvant-like properties. By conjugating fatty acid tails to peptide backbones, these hydrogels act as toll-like receptor (TLR) agonists, directly stimulating antigen-presenting cells (APCs) to enhance T-cell priming. The nanofibrous architecture of the hydrogel scaffold enables prolonged, sustained release of antigenic peptides, allowing for increased immune response duration and specificity. This approach addresses the current limitations of peptide-based cancer vaccines, particularly with regard to adjuvant efficacy and antigen retention. This research area aims to create a platform capable of inducing robust innate and adaptive immune responses, with applications extending beyond cancer vaccines to other immune-modulating therapies.

In Vitro Cancer Modelling
Crosslinkable hydrogels are used to mimic the extracellular matrices (ECM) of tumors to understand the roles of ECM mechanical properties and components on cancer cell phenotype changes, metastasis, and tumor progression. Here, we develop bio-inspired hydrogels that mimic the desmoplastic microenvironment characteristic of pancreatic ductal adenocarcinoma (PDAC). Using methacrylated hyaluronic acid (HAMA) and gelatin (GelMA)-based hydrogels, we can replicate the increased stiffness of the PDAC extracellular matrix (ECM) by tuning the crosslinking density. The result was a 3D in vitro model that enabled the epithelial-to-mesenchymal transition (EMT), a hallmark of cancer progression, providing a robust platform to study cancer-stroma interactions. By integrating cancer-associated fibroblasts (CAFs) and other stromal components into this bio-mimetic scaffold, we generated a biomimetic model for personalized drug testing and a deeper understanding of the mechanical and biochemical cues driving PDAC malignancy.

Futhermore, we developed a 3D in vitro model of Alzheimer’s disease (AD) using a multi-component peptide hydrogel to mimic the amyloid-fibril microenvironment associated with AD pathology. By engineering hydrogels that simulate both healthy brain ECM and amyloid plaques, we can culture neuronal progenitor cells (NPCs) and study their behavior in response to these differing environments. This model represents a significant advancement in biomaterial-based disease modeling, providing a platform for screening β-amyloid-targeting therapies and studying the cellular mechanisms underlying AD progression.

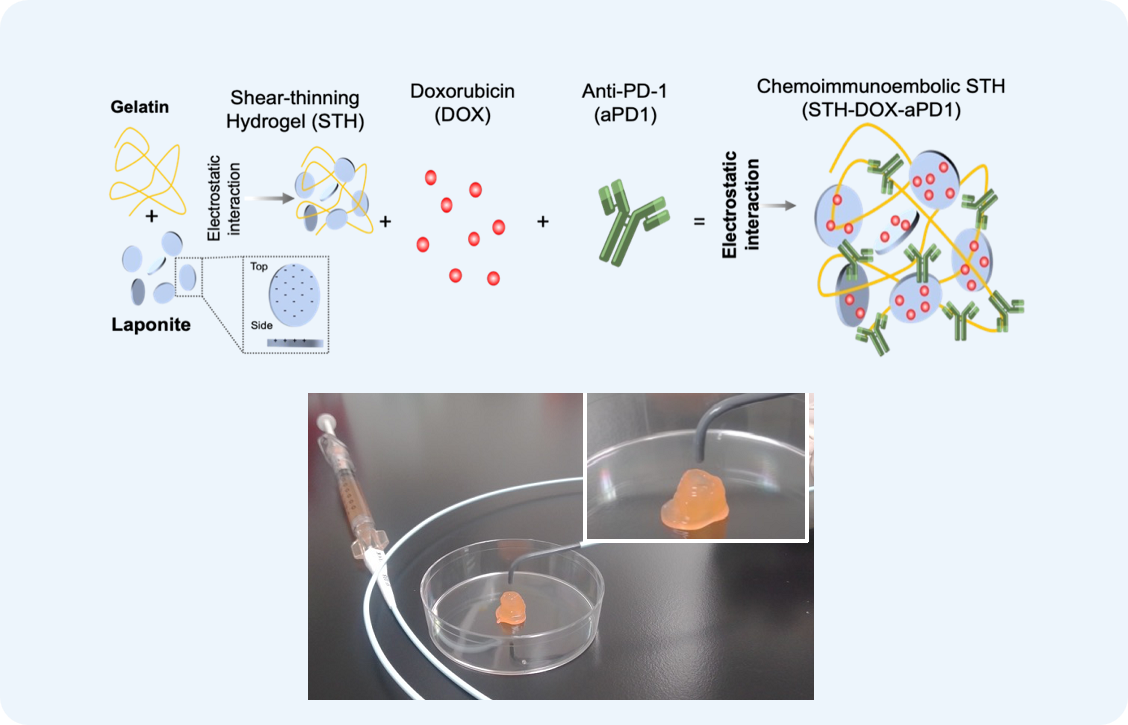
Shear-thinning Hydrogels for Drug Delivery
We design shear-thinning hydrogel systems for the localized and sustained delivery of immune checkpoint inhibitors (e.g., anti-PD-1) in combination with chemotherapeutic agents (e.g., doxorubicin) for the treatment of hepatocellular carcinoma (HCC), a deadly type of liver cancer. This gelatin-laponite hydrogel demonstrated significant promise in enhancing immune responses, reducing tumor burden, and minimizing recurrence rates in vivo. The material’s shear-thinning properties allowed for minimally invasive injection, providing targeted, site-specific release of therapeutic agents, and optimizing the tumor-immune interface. These hydrogels are an exciting step toward creating biomaterials with tailorable properties to improve therapeutic outcomes.

CONTACT US
For more information about our research, to discuss potential collaborations, or to inquire about joining our team, please reach out to us at nfalcone@terasaki.org.
RESEARCH PAPERS
Citations:660, H-index: 15
https://scholar.google.ca/citations?user=p7zGApcAAAAJ&hl=en
1. Mathes, T.G.; Esteves P.; Terasaki, M.; O’Raw, A.; Ermis, M.; Jucaud, V.; Khademhosseini, A.; Falcone, N.* Lipopeptide hydrogel possesses adjuvant-like properties for the delivery of the GPC-3 peptide-derived antigen. Advanced Functional Materials, 2024, Revisions Requested.
2. Mathes, T. G.; Monirizad, M.; Ermis, M.; Barros, N. R.; Rodriguez, M.; Kraatz, H. B.; Jucaud, V.; Khademhosseini, A.; Falcone, N.* Effects of amyloid-b-mimicking peptide hydrogel matrix on neuronal progenitor cell phenotype. Acta Biomaterialia. 2024, 183, 89-100.
3. Gangrade, A.; Zehtabi, F.; Rashad, A.; Haghniaz, R.; Falcone, N.; Mandal, K.; Khosravi, S.; Deka, S.; Yamauchi, A.; Voskanian, L.; Kim, H.; Ermis M.; Khademhosseini, A.; Barros, N. R. Nanobioactive blood-derived shear-thinning biomaterial for tissue engineering applications. Applied Materials Today. 2024, 38, 102250.
4. Biswas, S.; Lee, S. W.; Lee, Y.; Choi H.; Chen, J.; Yang, X.; Du, Y.; Falcone, N.; Barros, N. R.; Lee, S.; Kim, H.; Khademhosseini, A.; Zhu, Y. Emerging energy harvesters in flexible bioelectronics: From wearable devices to biomedical innovations. Small Science. 2024, 4, 2300148.
5. Barros, N.R.; Gangrade, A.; Rashad, A.; Chen, R.; Zehtabi, F.; Ermis, M.; Falcone, N.; Haghniaz R.; Khosravi, S.; Gomez, A.; Huang, S.; Mecwan, M.; Khorsandi, D.; Lee, J.; Zhu, Y.; Li, B.; Kim, H.; Thankam F. G.; Khademhosseini, A. Injectable nanoengineered adhesive hydrogel for treating enterocutaneous fistuals. Acta Biomaterialia. 2024, 173, 231-246.
6. Falcone, N.; Ermis, M.; Gangrade, A.; Choroomi, A.; Young, P.; Mathes, T. G.; Monirizad, M.; Zehtabi, F.; Mecwan, M.; Rodriguez, M.; Zhu, Y.; Byun, Y.; Khademhosseini, A.; Barros, N. R.; Kim, H. Drug-Eluting Shear-thinning Hydrogel for the Delivery of Chemo- and Immunotherapeutic Agents for the Treatment of Hepatocellular Carcinoma. Advanced Functional Materials. 2023, 2309069.
7. Falcone, N.*; Ermis, E.; Tamay, D. G.; Mecwan, M.; Monirizad, M.; Mathes, T. G.; Jucaud, V.; Choroomi, A.; Barros, N.; Zhu, Y.; Vrana, N. E.; Kraatz, H. B.; Kim, H.; Khademhosseini, A. Peptide hydrogels as immunomaterials and their use in cancer immunotherapy delivery. Advanced Healthcare Materials. 2023, 2301096.
8. Barros, N. R.; Wang, C.; Maity, S.; Peirsman, A.; Nasiri, R.; Herland, A.; Ermis, M.; Kawakita, S.; Carvalho, B. G.; Kouchehbaghi, N. H.; Herculano, R. D.; Tirpakova, Z.; Dabiri S. M. H.; Tanaka, J. L.; Falcone, N.; Choroomi, A.; Chen, R.; Huang, S.; Zisblatt, E.; Huang, Y.; Rashad, A.; Khorsandi, D.; Gangrade, A.; Voskanian, L. K.; Zhu, Y.; Li, B.; Akbari, M.; Lee, J.; Dokmeci, M. R.; Kim, H.; Khademhosseini, A. Engineering organoids for biomedical applications. Advanced Drug Delivery Reviews. 2023, 115142.
9. Ansari, M. A. A.; Dahs, M.; Camaci-Unal, G.; Jain, P. K.; Nukavarapu, S.; Ramakrishna, S.; Falcone, N.; Dokmeci, M. R.; Najafabadi, A. H.; Khademhosseini, A.; Nanda, H. S. Engineering stimuli-responsive smart grafts for bone regeneration. Current Opinion In Biomedical Engineering. 2023, 100493.
10. Barros, N.; Darabi, M.A.; Ma, X.; Diltemiz, S. E.; Ermis, M.; Najafabadi, A. H.; Nadine, S.; Banton, E. A.; Mandal, K.; Abbasgholizadeh, R.; Falcone, N.; Mano, J. F.; Nasiri, R.; Herculano, R. D.; Zhu, Y.; Ostrovidov, S.; Lee, J.; Kim, H.; Hosseini V.; Dokmeci, M. R.; Ahadian, S.; Khademhosseini, A. Enhanced Maturation of 3D Bioprinted Skeletal Muscle Tissue Constructs Encapsulating Soluble Factor-Releasing Microparticles. Macromolecular Bioscience. 2023, 2300276.
11. Barros, N. R.; Gomez, A.; Ermis, M.; Falcone, N.; Haghniaz, R., Young, P.; Gao, Y.; Aquino, A.; Li, S.; Niu, S.; Chen, R.; Huang, S.; Zhu, Y.; Eliahoo, P.; Sun, A.; Khorsandi, D.; Kim, J.; Kelber, J.; Khademhosseini, A.; Kim, H.; Li, B. Gelain methacryloyl and laponite bioink for 3D bioprinted organotypic tumor modeling. Biofabrifcation. 2023, DOI 10.1088/1758-5090/ace0db.
12. Ermis, M.;* Falcone, N.;* Barros, N.; Mecwan, M.; Haghniaz, R.; Choroomi, A.; Monirizad, M.; Lee Y.; Song, J.; Cho, H.; Zhu Y.; Kang, H.; Dokmeci, M. R.; Khademhosseini, A.; Lee, J.; Kim, H. Tunable hybrid hydrogels with multicellular spheroids for modeling desmoplastic pancreatic cancer. Bioactive Materials. 2023, 25, 360-373.
13. Falcone, N.; Mecwan, M.; Najafabadi, A. H.; Khorsandi, D. Chapter 4: Physicochemical and Mechanical Properties, ACS Symposium Series, 2023, 1438, DOI: 10.1021/bk-2023-1438.ch004.
14. Mecwan, M.; Falcone, N.; Najafabadi, A. H.; Khorsandi, D.; Chapter 5: Antioxidant Activity, ACS Symposium Series, 2023, 1438, DOI: 10.1021/bk-2023-1438.ch005
15. Zhu, Y.; Li, J.; Kim, J.; Li, S.; Zhao, Y.; Bahari, J.; Eliahoo, P.; Li, G.; Kawakita, S.; Haghniaz, R.; Gao, X.; Falcone, N.; Ermis, M.; Kang, H.; Liu, H.; Kim, H.; Tabish, T.; Yu, H.; Li, B.; Akbari, M.; Emaminejad, S.; Khademhosseini, A. Skin-interfaced electronics: A promising and intelligent paradigm for personalized healthcare. Biomaterials. 2023, 122075.
16. Zhu, Y.; Li, S.; Li, J.; Falcone, N.; Cui, Q.; Shah, S.; Hartel, M.; Yu, N.; Young, P.; Barros, N.; Wu, Z.; Haghniaz, R.; Ermis, M.; Kang, H.; Lee, J.; Karamikamkar, S.; Ahadian, S.; Jucaud, V.; Dokmeci, M.; Kim, H.; Khademhosseini, A. Lab-on-a-Contact Lens: Recent Advances and Future Opportunities in Diagnostics and Therapies. Advanced Materials, 2022, 2108389.
17. Mecwan, M.; Li, J.; Falcone, N.; Ermis, M.; Torres, M.; Morales, R.; Hassani, A.; Haghniaz, R.; Mandal, K.; Sharma, S.; Maity, S.; Zehtabi, F.; Zamanian, B.; Herculano, R.; Akbari, M.; John, J. V.; Khademhosseini, A. Recent Advances in biopolymer-based hemostatic materials. Regenerative Biomaterials. 2022. 9.
18. Lee, J.; Wang, Y.; Xue, C.; Chen, Y.; Qu, M.; Thakor, J.; Zhou, X.; Barros, N.; Falcone, N.; Young, P.; van den Dolder, F. W.; Lee, K.; Zhu, Y.; Cho, H-J.; Sun, W.; Zhao, B.; Ahadian, S.; Jucaud, V.; Dokmeci, M. R.; Khademhosseini, A.; Kim, H. pH-responsive Doxorubicin Delivery using Shear-Thinning Biomaterials for Localized Melanoma Treatment. Nanoscale. 2021,14, 350-360.
19. Falcone, N.; Andoy, N. M. O.; Sullan, R. M. A.; Kraatz, H. B.; Peptide-polydopamine Hybrid Hydrogel for a LASER-controlled Hydrophobic Drug Delivery, ACS Applied Bio Materials, 2021, 4, 6652-6657.
20. Barros N R, Chen Y, Hosseini V, Wang W, Nasiri R, Mahmoodi M, Yalcintas E P, Haghniaz R, Mecwan, M. M.; Karamikamkar, S.; Dai, W.; Sarabi, S. A.; Falcone, N.; Young, P.; Zhu, Y.; Sun, W.; Zhang, S.; Lee, J.; Lee, K.; Ahadian, S.; Dokmeci, M. R.; Khademhosseini, A.; Kim, H. Recent Developments in Mussel-Inspired Materials for Biomedical Applications. Biomaterials Science. 2021 9, 6653-6672.
21. Li, X.; Falcone, N.; Hossain, M. N.; Kraatz, H.-B.; Huang, H. Development of a novel label- free impedimetric electrochemical sensor based on hydrogel/chitosan for the detection of ochratoxin A. Talanta, 2021, 226, 122183.
22. Falcone, N.; Shao, T,; Andoy, N.; Rashid, R.; Sullan, R. M. A.; Sun, X.; Kraatz, H.-B. Multi-component Peptide Hydrogels – A Systematic Study Incorporating Biomolecules for the Exploration of Diverse Tuneable Biomaterials, Biomaterials Science, 2020, 8, 5601-5614. *Selected as a HOT article in Biomaterials Science Recent HOT Articles, also chosen as the outer front cover of the journal*
23. Shao, T.; Falcone, N.; Kraatz, H-.B. Supramolecular Gels: Influencing Properties by Metal Ion Coordination and Their Wide-Ranging Applications, ACS Omega, 2020, 5, 1312-1317.
24. Falcone, N.; Shao, T.; Rashid, R.; Kraatz, H.-B. Enzyme Entrapment in Amphiphilic Myristyl-Phenylalanine Hydrogels, Molecules, 2019, 245, 2884-2901.
25. Basak, S.; Falcone, N.; Ferranco, A.; Kraatz, H-B. Remarkable morphology transformation from fiber to nanotube of a Histidine organogel in presence of a binuclear Iron(III)-sulfure complex, J. Inorg. Organ. Polymers. Mater., 2019, 1-10.
26. Luka, G., Ahmad, S., Falcone, N., Kraatz, H.-B. Enzyme-based Electrochemical Sensors: Current Trends, Benefits, and Constraints, Bioelectronics and Medical Devices, 2019, 555-590.
27. Falcone, N., Kraatz, H.-B. Supramolecular Assembly of Peptide and Metallopeptide Gelators and Their Stimuli-Responsive Properties in Biomedical Applications, Chem. Eur. J., 2018, 24, 14316-14328.
28. Falcone, N., Syed, She, Z., J., Lough, A., Kraatz, H. -B. Synthesis and Biochemical Evaluation of Three Novel Nicotinamide Derivatives as NADH analogues, ChemBioChem, 2018, 20, 838-845.
29. Falcone, N., Shao, T., Sun, X., Kraatz, H.-B. Systematic exploration of the pH dependence of a peptide hydrogel, Can. J. Chem., 2018, 97, 430-434.
30. Falcone, N., Kraatz, H.-B. Ferrocene Peptide-based Supramolecular Gels: Current Trends and Applications, Advances in Bioorganometallic Chemistry, 2018, (Hirao-ABC-1631464, Chpt 0003)
31. Falcone, N., Basak, S., Dong, B., Syed, J., Ferranco, A., Lough, A., She, Z., Kraatz, H.-B. Ferrocene-Tryptophan Conjugate: Role of Indolic Nitrogen in Supramolecular Assembly, ChemPlusChem, 2017, 82, 1282 –1289.
32. Falcone, N., She, Z., Chen, C., Dong, B., Yi, D., Kraatz, H.-B. Demonstration of a tailorable and PCR-free detection of Enterococcus DNA isolated from soil samples. Anal. Meth., 2017, 9, 1643-1649.















