July 13, 2020
Component of tissue is shown to limit the amount of scarring after heart attack.
(LOS ANGELES) – When a person has a heart attack, the person’s coronary artery is blocked, cutting off the flow of blood and oxygen to that portion of the person’s heart. The surrounding heart muscle may be damaged to an extent depending on the size of the blocked area and the time between the attack and treatment. Upon recovery, the heart muscle starts to heal, and like a skin wound, it may form a scar. The size and location of the scar can vary greatly, and there is a possibility to develop additional complications or even death.
Although there are potential problems associated with cardiac scarring, little is known about the factors involved in regulating cardiac scar size after a heart attack. An understanding and demonstration of the role such factors play in the scarring process may result in new medical treatments.
As recently published in the journal Cell, a collaborative group including Ali Khademhosseini, Ph.D. and Samad Ahadian, Ph.D., of the Terasaki Institute for Biomedical Innovation (TIBI), has identified such a factor: collagen V. Collagen is the most abundant protein in the human body and is found in bones, tendons, muscles, skin, and various internal organs. It provides structure and strength and holds the body together. It is also a component of scar tissue, which can be formed in various places throughout the body.
Within a healthy heart, there are different types of collagen, but collagen V is found in only small quantities. However, the group observed that large quantities of collagen V were found in cardiac injury scars. Previous independent studies of animals, genetically deficient for the production of collagen V, had shown increased cardiac scar tissue. These observations suggested that collagen V might somehow be involved in scar formation.
The group prepared mice that were completely deficient in producing collagen V and performed several experiments on them to test the effects of collagen V. Their findings indicate that upon cardiac injury equivalent to a heart attack, the genetic activity of the collagen V-deficient mice favored the formation of larger and more irregularly-constructed cardiac scars than that of normal control mice. In addition, fibroblasts, specialized cells that produce collagen and are part of the matrix that makes up scar tissue, exhibited different behavioral and mechanical properties in the collagen V-deficient mice compared to normal mice. During the normal healing process following cardiac injury, cardiac fibroblasts exert forces upon cardiac scars and cause them to shrink.
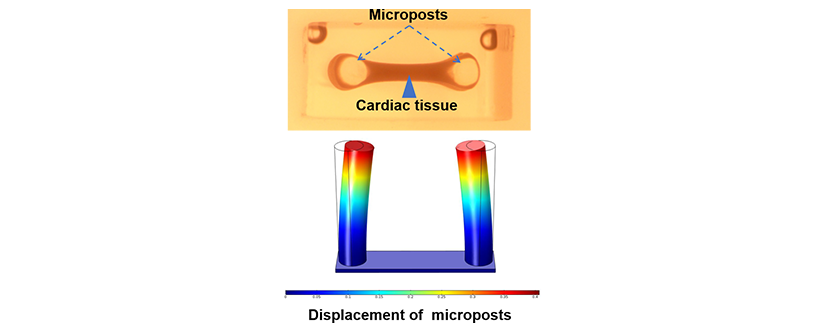
The team hypothesized that since the cardiac scar tissue without collagen V is larger, stiffer, and irregularly structured, the forces exerted by its fibroblasts would also be less. In order to test the hypothesis, the team made two separate scaffold preparations, consisting of a collagen hydrogel mixed with cardiac injury fibroblasts from either a normal or collagen V-deficient mouse. The scaffolds were then attached to two silicon-based posts and observed for the displacement of the posts due to fibroblast contraction. It was found that there was significantly less displacement of the posts with the collagen V-deficient fibroblasts than the normal fibroblasts, thus confirming their hypothesis and providing evidence for larger scar formation without collagen V. “By being able to fabricate and characterize microtissues, we could measure mechanical forces of heart tissues in a dish. Similar approaches can be used for human studies in a personalized manner,” said Samad Ahadian, Ph.D., lead investigator of the Terasaki Institute team. As collagen V expression can vary among individuals, the elucidation of the feedback mechanisms and interactions of collagen V in scar formation may have profound implications for cardiac patients who suffer from the various heart ailments associated with cardiac injury scars.
Such knowledge may also have an even broader influence beyond mitigating cardiac ailments. Individuals with Ehlers Danlos Syndrome have genetic mutations that leave them deficient in collagen V production, and they exhibit abnormalities in the connective tissues of their joints, skin, and other tissues. They also exhibit abnormal wound healing with increased scar sizes. Experiments have shown that fibroblasts from individuals with this syndrome have increased expression of a protein called integrin, due to their lack of collagen V production; these individuals could possibly benefit from therapies involving inhibitors of integrin signaling.
“Understanding biological mechanisms that control tissue behavior is crucial for developing personalized therapeutics. The work here demonstrates the importance of matrix molecules in cardiac tissue regeneration and scar formation,” said Khademhosseini, Director and CEO of Terasaki Institute. “Therefore, this work is of considerable significance in developing approaches that induce tissue healing. As such, this work synergizes with a number of Platforms at the Terasaki Institute, which aim to leverage our ability to find personalized solutions for patients.”