(LOS ANGELES) – An extensive analytical study conducted at the Terasaki Institute for Biomedical Innovation (TIBI) has revealed an association between favorable survival outcomes for melanoma patients and the presence of higher populations of tissue-resident memory T cells (TRM). Data obtained from this study could be used not only for a TRM-based machine learning model with predictive powers for melanoma prognosis but could also elucidate the role TRM cells can play in the tumor immune microenvironment. This could guide the development of more effective and personalized anti-tumor immunotherapeutic treatment regimens for cancer patients.
The tumor immune microenvironment (TIME) refers to the complex and dynamic interplay between tumor cells, various immune cells, and other cellular and non-cellular components within and surrounding the tumor.
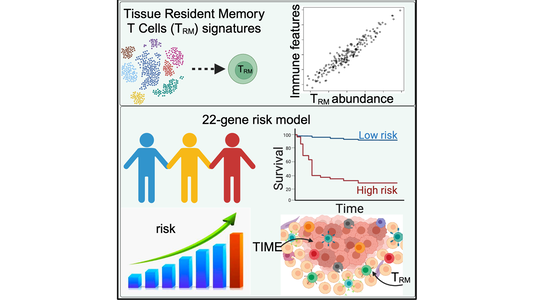
TRM cells are a unique type of immune cells that reside in peripheral tissues and many kinds of cancer types.
Because of the presence and functional properties of TRM cells within the TIME in harnessing their potential for cancer immunotherapy, there has been much interest in studying TRM cells and how they influence patient survival. Key to this understanding is to establish whether the presence and abundance of TRM cells in cancer patients correlate with better patient prognosis. Previous studies conducted with melanoma patients have produced conflicting results. There has also been little effort to conduct a comprehensive study to evaluate the TRM abundance and correlate immunomics data with patient survival outcomes.
The TIBI team sought to solve this problem by turning to data from single-cell RNA sequencing (scRNA-seq), a powerful technology that allows one to obtain a complete genetic profile of large numbers of individual cells. Instead of using a limited number of a cell’s identifying marker genes, utilizing the scRNA-seq technology provides a more comprehensive, accurate, and nuanced way of characterizing a cell’s type and function. From this profile, gene signatures can be generated– uniquely characteristic patterns of a specific immune cell type that can possibly be correlated with the presence of disease.
As described in their recent paper in iScience, the team used this approach on two independent cohorts of melanoma patients' scRNA-seq data and were able to extract 11 distinct gene signatures that highly correlated with TRM abundance in the patients. A solid association was also found between these gene signatures and patient survival outcomes.
Further studies revealed additional positive correlations between TRM abundance and the presence of multiple anti-tumor immune cells in the melanoma TIME, as well as with immune pathways and regulatory genes, suggesting that TRM cells have a crucial role in immunomodulation. The studies also indicated that an abundance of TRM cells results in a more active melanoma TIME and better patient outcomes.
Finally, the TIBI researchers could use the data from their analysis to create a high-precision TRM-derived risk scoring system to classify patients into high- and low-risk prognostic categories for melanoma patients.
“Our scientists’ analytical approach and discoveries about the role that TRM cells play may help to refine and more accurately assess cancer patients’ response to immunotherapeutic drugs,” said Ali Khademhosseini, TIBI’s Director and CEO. “As treatments can have profoundly variable effects on individual cancer patients, this is an important step toward improving patient outcome.”