New hydrogel features enhanced capabilities for treating aneurysms and halting their progression
(LOS ANGELES) – A novel, injectable shear-thinning hydrogel for treating abdominal aortic aneurysms (AAA) has been developed by scientists at the Terasaki Institute for Biomedical Innovation (TIBI). This hydrogel not only demonstrates increased effectiveness at sealing off blood flow to AAAs, but also stops the growth of the aneurysms themselves.
Aneurysms, which can occur in blood vessels in many areas of the body, are due to atherosclerosis (plaque buildup in the vessels), infections or other inflammatory diseases and risk factors. Chronic inflammation from these conditions causes endothelial cells, which form the lining inside the blood vessels, to secrete excessive levels of enzymes called matrix metalloproteinases (MMPs). These MMPs degrade fibers in the vessel walls, and the weakened areas can balloon outward, with possible fatal rupture. Aneurysms in the aorta, the body’s largest artery, is the second most common aortic disease and the ninth leading cause of death worldwide.
Aneurysm patients who are feeling pain or other symptoms, or whose aneurysms are growing at accelerated rates are advised to seek treatment, and minimally invasive treatments are especially recommended for those with comorbidities or vulnerabilities to open surgical procedures.
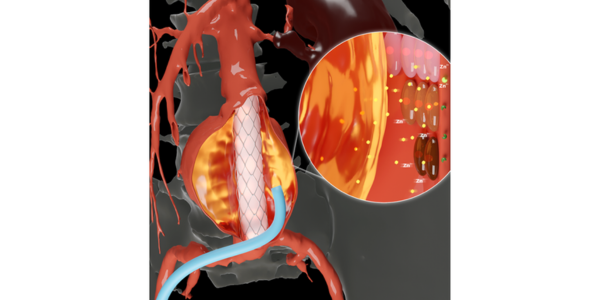
The standard non-invasive treatment for patients with aneurysms is to insert a stent graft, a mesh-lined tube, into the affected blood vessel to shore up its walls and block blood flow to the aneurysm. The method is not foolproof, however, because leaks can occur between the stent graft and the endothelial wall, allowing blood to feed once again into the aneurysm.
To counteract these so-called endoleaks, blocking or embolic agents are applied to the aneurysm site. However, these commercial agents can be costly, difficult to handle, and ineffective against endoleaks, necessitating repeat treatment procedures.
The TIBI scientists developed a novel embolic agent for AAA that would not only improve its effectiveness against endoleaks but would also suppress further progression of aneurysm growth. They began by formulating a gelatin-based shear-thinning hydrogel, an injectable gel that quickly self-solidifies after injection, which contained silicate nanoplatelets to enhance cohesion and blood clotting capabilities.
They then introduced a new component, doxycycline (DOX), which is FDA approved for its ability to remove dysfunctional endothelial cells and to inactivate any secreted MMPs. These effects by DOX are facilitated both by its ability for quick burst release for rapid removal of endothelial cells and sustained release for longer-term MMP inhibition. The TIBI scientists demonstrated this two-step process through animal experiments using their new hydrogel in samples of pig aorta.
In separate experiments, the scientists were also able to determine that the addition of doxycycline resulted in a hydrogel with even greater injectability, cohesion, and embolic strength, exhibiting a 33% reduction in clotting time. Additionally, when using their new hydrogel, they achieved successful embolization in a benchtop endoleak model and showed that DOX affects the expression of several proteins involved in endothelial cell function, blood vessel formation, and blood coagulation.
These findings open exciting possibilities to adapt the new DOX hydrogel to treat aneurysms in different locations in the body, as well as to develop further experiments to test their long-term safety and efficacy.
“The work here represents a significant advancement in the treatment of aneurysms,” said Ali Khademhosseinini, Ph.D., TIBI’s Director and CEO. “It is our hope that this work can be expanded into further tests and clinical trials so that we may bring this technology to the patients.”