(LOS ANGELES) – Pancreatic ductal adenocarcinoma (PDAC), is highly aggressive and lethal. It is the most prevalent type of pancreatic cancer, making up 90% of cases; it also has a high rate of metastasis, with an average five-year survival rate of less than 10%.
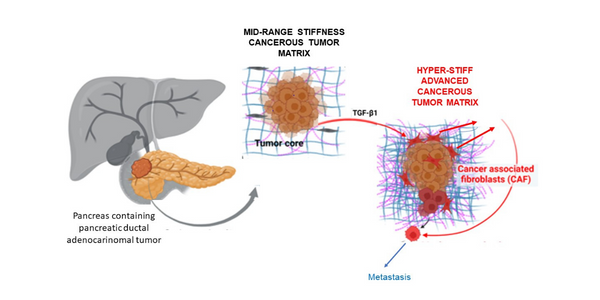
It is thought that the dense, stiff matrix immediately surrounding its tumor cells plays a major role in PDAC disease progression. In addition to influencing pancreatic tissue fibrosis, it also limits accessibility and effectiveness of anticancer drugs against the tumor and enhances the promotion of surface protrusions on the PDAC cancer cells, increasing the potential for metastasis.
Because of the complexity and interactivity of the various matrix components surrounding PDAC tumors, it is difficult to design fully accurate and representative models for study. However, scientists from the Terasaki Institute for Biomedical Institute (TIBI) have recently designed an advanced pancreatic tumor model which more accurately depicts PDAC tumor progression and can test the effects of its stiff, fibrous matrix on pancreatic tissue fibrosis.
Eighty percent of the microenvironment surrounding PDAC tumors is composed of a supportive and fibrous connective tissue matrix, which is largely composed of collagens and cellular components such as pancreatic cancer cells, immune cells, and collagen-producing, cancer-associated fibroblast (CAF) cells. All of these components interactively affect pancreatic tumor progression.
The TIBI team’s approach uses pancreatic cancer cells and CAFs to form three-dimensional cellular aggregates called spheroids, and to embed these spheroids into a hybrid hydrogel formulation. This hybrid mixture contained components that are elevated in the PDAC matrix and could be experimentally tuned to different degrees of stiffness. Such a model could closely mimic PDAC tissues and offer a means of testing the effects of tissue stiffness and other factors on tumor progression.
In subsequent tests using their model, the scientists were able to observe the influence of CAFs on cell-to-cell interaction, as manifested in the more compact shape of the CAF-containing spheroids. These influences, in turn, promoted the pancreatic cancer cells’ viability.
Additional experiments demonstrated the effect of varying levels of matrix stiffness on PDAC disease progression. The researchers tuned their hybrid hydrogel to stiffness levels representing a healthy state, a mid-range stiffness matrix, and a hyper-stiff, advanced cancerous state matrix. They then measured the spheroid-produced biomarkers that were influenced by the mechanical effects of these stiffness levels.
These biomarkers, either promoters or indicators of tumor progression, were produced at high levels when the hybrid hydrogels were tuned to hyper-level matrix stiffness. Moreover, it was also found that these biomarker levels could be increased in hybrid hydrogels with mid-range matrix stiffness by the addition of TGF-beta, a growth factor that plays key roles in cancer progression.
The results of the experiments suggest that TGF-beta influences on tumor progression from tissues in a mid-range matrix stiffness level can override the mechanical influences that govern tumor progression in hyper-level matrix stiffnesses.
“In a uniquely aggressive and difficult-to-treat disease such as pancreatic ductal adenocarcinoma, it is of utmost importance that we understand the interactive factors that influence its progression,” said Ali Khademhosseini, Ph.D., TIBI’s Director and CEO. “Designing advanced physiological models as we have here is an important step in achieving this and may lead to the development of personalized modeling and much-needed drug testing applications.”
Authors are: Menekse Ermis, Natashya Falcone, Natan Roberto de Barros, Marvin Mecwan, Reihaneh Haghniaz, Auveen Choroomi, Mahsa Monirizad, Yeji Lee, Jihyeon Song, Hyun-Jong Cho, Yangzhi Zhu, Heemin Kang, Mehmet R. Dokmeci, Ali Khademhosseini, Junmin Lee, Han-Jun Kim.
This work was supported in part by the National Institutes of Health (HL140951, HL137193, CA257558, DK130566).
DOI: https://doi.org/10.1016/j.bioactmat.2023.02.005