Home
Vadim Jucaud Laboratory
About Dr. Vadim Jucaud

Dr. Vadim Jucaud is an Assistant Professor at the Terasaki Institute for Biomedical Innovation (TIBI). Dr. Jucaud has been part of the Terasaki Laboratory since 2010, where he started his research career under the mentorship of Professor Paul I. Terasaki in the fields of histocompatibility and immunogenetics, with a particular emphasis on HLA antibodies and the Humoral Theory of Transplantation. Dr. Jucaud has a Ph.D. in Immunology and Microbiology, specializing in Human Leukocyte Antigen (HLA) immunobiology, notably in the diversity, detection, and prediction of the humoral response against HLA molecules. Dr. Jucaud is an expert in HLA immunogenetics, histocompatibility, and immunogenicity. In addition, he has excellent technical skills forged by 12 years of experience in characterizing the humoral immune response in transplant patients, T and B cell immunomodulation, sensitive multiplex immunoassays, antibody cross-reactivity and immunogenic antibody epitopes, and HLA antibody/antigen interactions. Dr. Jucaud received the American Transplant Congress Young Investigator Award in 2018 for his outstanding work demonstrating the prevalence and impact of de novo donor-specific antibodies during a multicenter immunosuppression withdrawal trial in adult liver transplant recipients.
With the establishment of TIBI in 2020 by Dr. Ali Khademhosseini, Distinguished Professor and CEO, Dr. Jucaud has been leading the organ-on-a-chip and biosensors team, Lastly, Dr. Jucaud has authored more than 30 peer-reviewed publications and 28 conference abstracts, and he has an h-index of 13 with more than 463 citations.Research Overview
Dr. Vadim Jucaud’s Lab focuses on developing immunocompetent organ-on-a-chip models and microphysiological systems for disease modeling and drug screening applications.
Organs-on-a-Chip and Microphysiological System
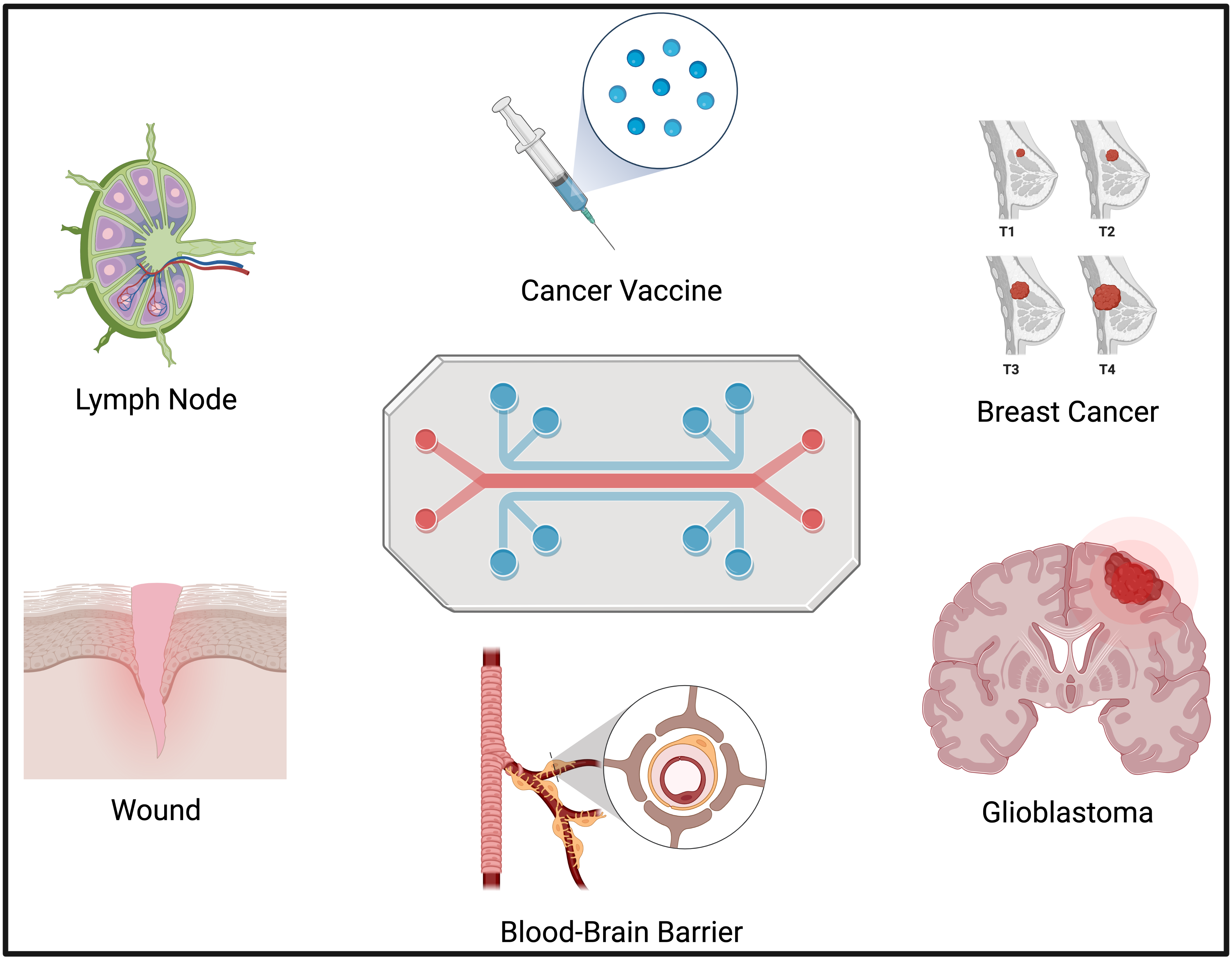
Immunocompetent Models
- Lymph node on-a-chip
- Cancer vaccine on-a-chip
Microvasculature Models
- Breast cancer on-a-chip
- Glioblastoma on-a-chip
- Blood-brain barrier on-a-chip
Disease States Models
- 3D multicellular glioblastoma model
- Wound on-a-chip
Transplant Immunology
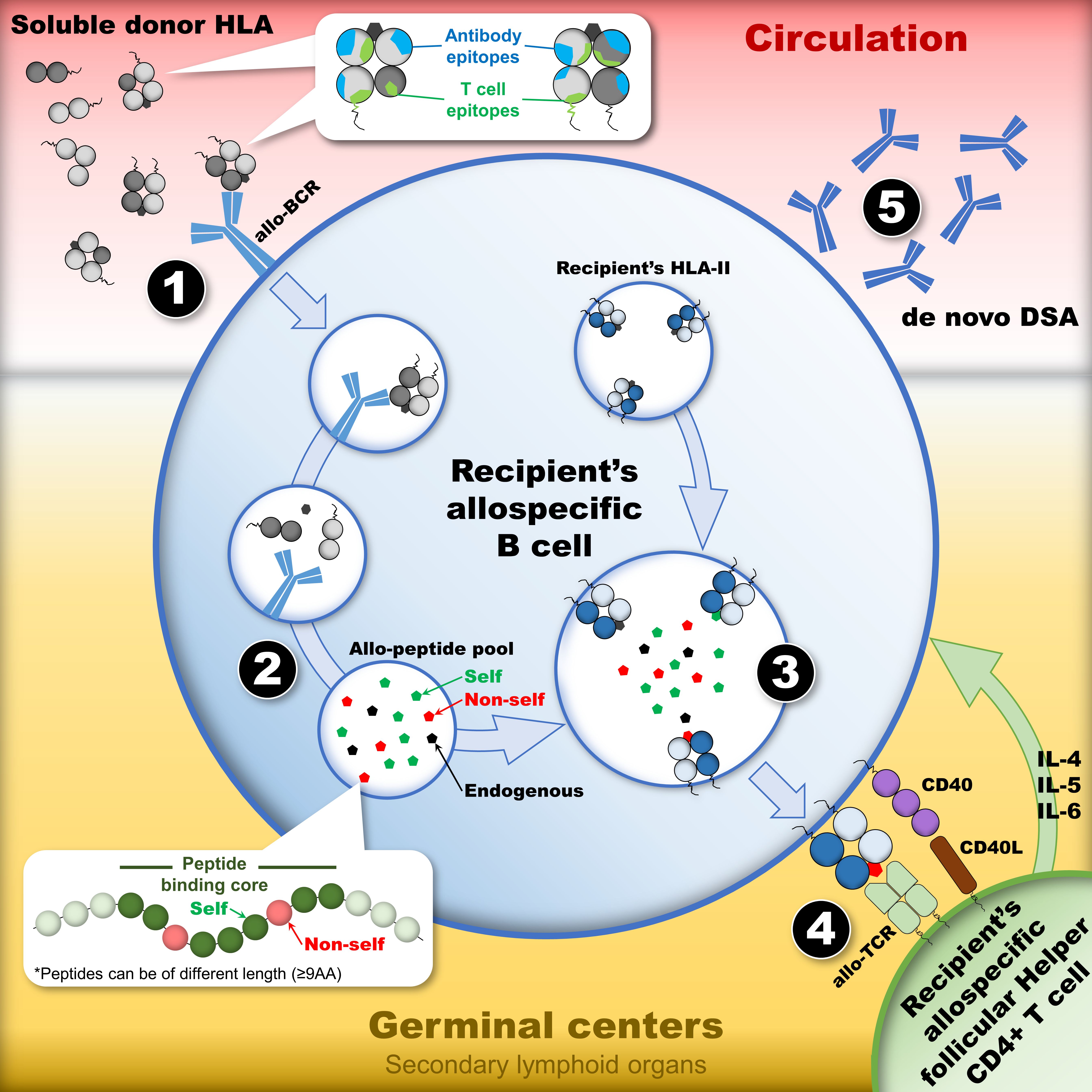
HLA Immunogenicity
- HLA antibodies
- HLA epitopes
Transplant Tolerance
Biomaterials
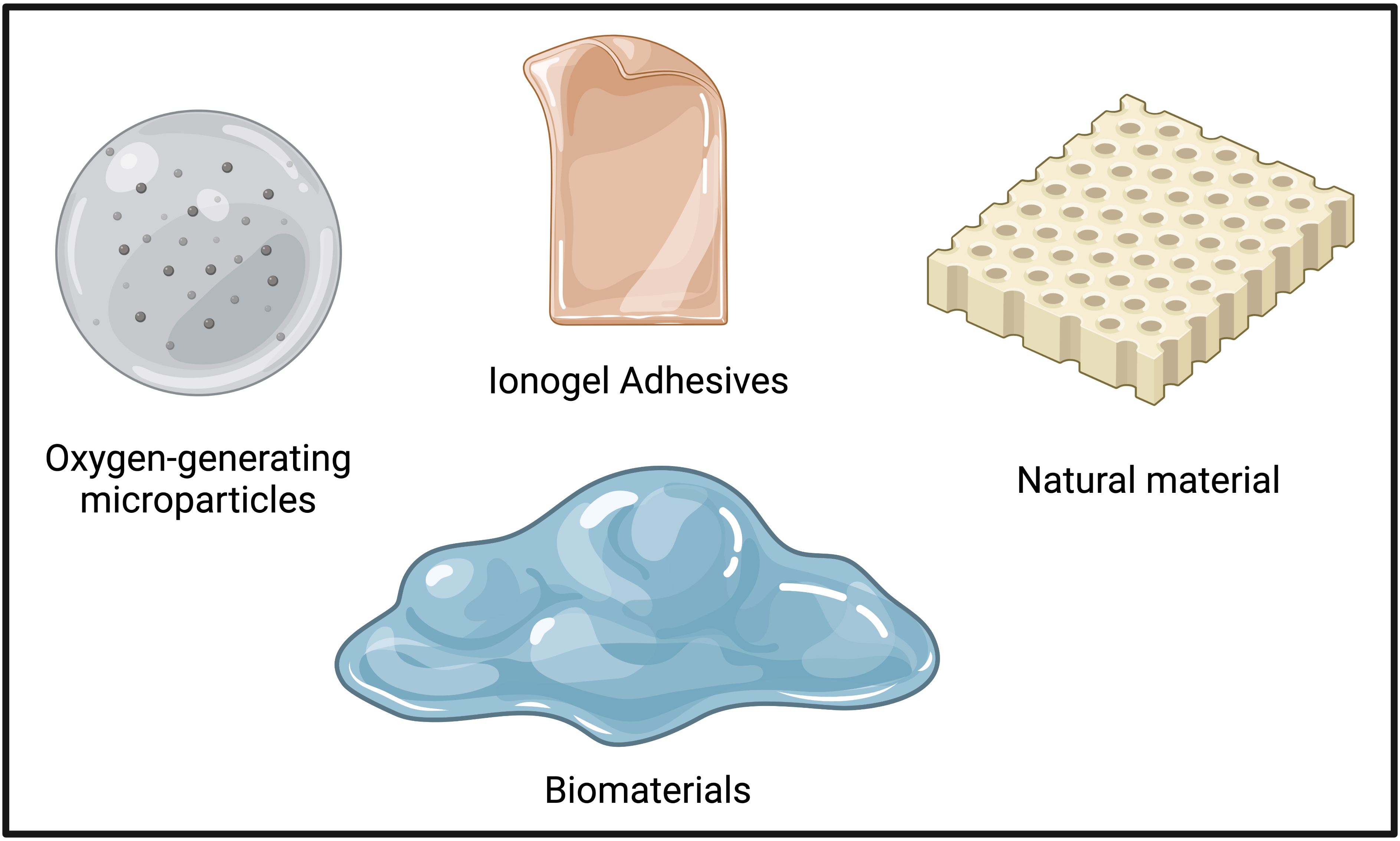
Oxygen-Releasing Microparticle
Ionogel Adhesive
Natural Rubber Latex
Biosensors and biomarker detection
Micro Cell-Based ELISA
Exosome Detection and Quantification
Team Members
Dr. Jucaud’s lab consists of a highly interdisciplinary research team specialized in HLA immunobiology, organ-on-a-chip technologies, tissue engineering, biomaterials, and biosensors.
Postdoctoral Researchers
.jpg)
Surjendu Maity
.jpg)
Arne Peirsman
.jpg)
Huu Tuan Nguyen
 Donizetti Herculano (1).jpg)
Rondinelli Herculano

Satoru Kawakita
Interns
.jpg)
Can Yilgor

Christopher Jewell
.jpg)
Christian Umemura
Alumni
.jpg)
Lei Mou
.jpg)
Gang Ge
 Li (1).jpg)
Jacky Li
Publications
1. Zhu Y, Li S, Li J, Falcone N, Cui Q, Shah S, Hartel MC, Yu N, Young P, de Barros NR, Wu Z, Haghniaz R, Ermis M, Wang C, Kang H, Lee J, Karamikamkar S, Ahadian S, Jucaud V, Dokmeci MR, Kim H-J, Khademhosseini A. Lab-on-a-Contact Lens: Recent Advances and Future Opportunities in Diagnostics and Therapeutics. Advanced Materials. 2022;34(24):2108389. doi: https://doi.org/10.1002/adma.202108389.
2. Zhu Y, Hartel MC, Yu N, Garrido PR, Kim S, Lee J, Bandaru P, Guan S, Lin H, Emaminejad S, de Barros NR, Ahadian S, Kim H-J, Sun W, Jucaud V, Dokmeci MR, Weiss PS, Yan R, Khademhosseini A. Epidermis-Inspired Wearable Piezoresistive Pressure Sensors Using Reduced Graphene Oxide Self-Wrapped Copper Nanowire Networks. Small Methods. 2022;6(1):2100900. doi: https://doi.org/10.1002/smtd.202100900.
3. Lee J, Wang Y, Xue C, Chen Y, Qu M, Thakor J, Zhou X, Barros NR, Falcone N, Young P, van den Dolder FW, Lee K, Zhu Y, Cho H-J, Sun W, Zhao B, Ahadian S, Jucaud V, Dokmeci MR, Khademhosseini A, Kim H-J. pH-Responsive doxorubicin delivery using shear-thinning biomaterials for localized melanoma treatment. Nanoscale. 2022;14(2):350-60. doi: 10.1039/D1NR05738C.
4. Zhu Y, Mandal K, Hernandez AL, Kawakita S, Huang W, Bandaru P, Ahadian S, Kim H-J, Jucaud V, Dokmeci MR, Khademhosseini A. State of the art in integrated biosensors for organ-on-a-chip applications. Current Opinion in Biomedical Engineering. 2021;19:100309. doi: https://doi.org/10.1016/j.cobme.2021.100309.
5. Zhu Y, Kim S, Ma X, Byrley P, Yu N, Liu Q, Sun X, Xu D, Peng S, Hartel MC, Zhang S, Jucaud V, Dokmeci MR, Khademhosseini A, Yan R. Ultrathin-shell epitaxial Ag@Au core-shell nanowires for high-performance and chemically-stable electronic, optical, and mechanical devices. Nano Research. 2021;14(11):4294-303. doi: 10.1007/s12274-021-3718-z.
6. Zhu Y, Haghniaz R, Hartel MC, Mou L, Tian X, Garrido PR, Wu Z, Hao T, Guan S, Ahadian S, Kim H-J, Jucaud V, Dokmeci MR, Khademhosseini A. Recent Advances in Bioinspired Hydrogels: Materials, Devices, and Biosignal Computing. ACS Biomaterials Science & Engineering. 2021. doi: 10.1021/acsbiomaterials.1c00741.
7. Nasrollahi F, Haghniaz R, Hosseini V, Davoodi E, Mahmoodi M, Karamikamkar S, Darabi MA, Zhu Y, Lee J, Diltemiz SE, Montazerian H, Sangabathuni S, Tavafoghi M, Jucaud V, Sun W, Kim H-J, Ahadian S, Khademhosseini A. Micro and Nanoscale Technologies for Diagnosis of Viral Infections. Small. 2021;17(45):2100692. doi: https://doi.org/10.1002/smll.202100692.
8. Ma X, Ahadian S, Liu S, Zhang J, Liu S, Cao T, Lin W, Wu D, de Barros NR, Zare MR, Diltemiz SE, Jucaud V, Zhu Y, Zhang S, Banton E, Gu Y, Nan K, Xu S, Dokmeci MR, Khademhosseini A. Smart Contact Lenses for Biosensing Applications. Advanced Intelligent Systems. 2021;3(5):2000263. doi: https://doi.org/10.1002/aisy.202000263.
9. Zhou X, Jiang X, Qu M, Aninwene GE, Jucaud V, Moon JJ, Gu Z, Sun W, Khademhosseini A. Engineering Antiviral Vaccines. ACS Nano. 2020;14(10):12370-89. doi: 10.1021/acsnano.0c06109.
10. Kawakita S, Beaumont JL, Jucaud V, Everly MJ. Personalized prediction of delayed graft function for recipients of deceased donor kidney transplants with machine learning. Scientific Reports. 2020;10(1):18409. doi: 10.1038/s41598-020-75473-z.
11. Jucaud V, Nguyen A, Tran B, Hopfield J, Pham T. Validation and cross-reactivity pattern assessment of monoclonal antibodies used for the screening of donor-specific IgG antibody subclasses in transplant recipients. Journal of Immunological Methods. 2020;486:112847. doi: https://doi.org/10.1016/j.jim.2020.112847.
12. Chen Y, Zhang S, Cui Q, Ni J, Wang X, Cheng X, Alem H, Tebon P, Xu C, Guo C, Nasiri R, Moreddu R, Yetisen AK, Ahadian S, Ashammakhi N, Emaminejad S, Jucaud V, Dokmeci MR, Khademhosseini A. Microengineered poly(HEMA) hydrogels for wearable contact lens biosensing. Lab on a Chip. 2020;20(22):4205-14. doi: 10.1039/D0LC00446D.
13. Jucaud V, Shaked A, DesMarais M, Sayre P, Feng S, Levitsky J, Everly MJ. Prevalence and Impact of De Novo Donor-Specific Antibodies During a Multicenter Immunosuppression Withdrawal Trial in Adult Liver Transplant Recipients. Hepatology. 2019;69(3):1273-86. doi: https://doi.org/10.1002/hep.30281.
14. Ravindranath MH, Jucaud V, Ferrone S. Monitoring native HLA-I trimer specific antibodies in Luminex multiplex single antigen bead assay: Evaluation of beadsets from different manufacturers. J Immunol Methods. 2017;450:73-80. Epub 2017/08/08. doi: 10.1016/j.jim.2017.07.016. PubMed PMID: 28782523.
15. Ravindranath MH, Jucaud V, Banuelos N, Everly MJ, Cai J, Nguyen A, Terasaki PI. Nature and Clonality of the Fluoresceinated Secondary Antibody in Luminex Multiplex Bead Assays Are Critical Factors for Reliable Monitoring of Serum HLA Antibody Levels in Patients for Donor Organ Selection, Desensitization Therapy, and Assessment of the Risk for Graft Loss. J Immunol. 2017;198(11):4524-38. Epub 2017/05/10. doi: 10.4049/jimmunol.1700050. PubMed PMID: 28476933.
16. Jucaud V, Ravindranath MH, Terasaki PI. Conformational Variants of the Individual HLA-I Antigens on Luminex Single Antigen Beads Used in Monitoring HLA Antibodies: Problems and Solutions. Transplantation. 2017;101(4):764-77. Epub 2016/08/09. doi: 10.1097/TP.0000000000001420. PubMed PMID: 27495776.
17. Jucaud V. The Immunogenicity of HLA Class II Mismatches: The Predicted Presentation of Nonself Allo-HLA-Derived Peptide by the HLA-DR Phenotype of the Recipient Is Associated with the Formation of DSA. J Immunol Res. 2017;2017:2748614. Epub 2017/03/24. doi: 10.1155/2017/2748614. PubMed PMID: 28331856; PMCID: PMC5346368 of this paper.
18. Hilali FE, Jucaud V, Hilali HE, Bhuiyan MH, Mancuso A, LiuSullivan N, Elidrissi A, Mazouz H. Characterization of the Anti-HLA Class I and II IgG Antibodies in Moroccan IVIg Using Regular Beads and Ibeads in Luminex Multiplex Single Antigen Immunoassay. International Journal of Immunology. 2017;5(4):53-65.
19. El-Awar N, Jucaud V, Nguyen A. HLA Epitopes: The Targets of Monoclonal and Alloantibodies Defined. J Immunol Res. 2017;2017:3406230. Epub 2017/06/20. doi: 10.1155/2017/3406230. PubMed PMID: 28626773; PMCID: PMC5463109.
20. Ravindranath MH, Jucaud V, Maehara CY, Terasaki PI. Significance of the differences in the prevalence of anti-HLA antibodies in matched pairs of mother’s and cord blood. Immunology Letters. 2016;170:68-79. doi: https://doi.org/10.1016/j.imlet.2015.11.016.
21. Jucaud V, Ravindranath MH, Terasaki PI, Morales-Buenrostro LE, Hiepe F, Rose T, Biesen R. Serum antibodies to human leucocyte antigen (HLA)-E, HLA-F and HLA-G in patients with systemic lupus erythematosus (SLE) during disease flares: Clinical relevance of HLA-F autoantibodies. Clinical and Experimental Immunology. 2016;183(3):326-40. doi: 10.1111/cei.12724.
22. Ravindranath MH, Terasaki PI, Pham T, Jucaud V. The Monospecificity of Novel Anti-HLA-E Monoclonal Antibodies Enables Reliable Immunodiagnosis, Immunomodulation of HLA-E, and Upregulation of CD8+ T Lymphocytes. Monoclon Antib Immunodiagn Immunother. 2015;34(3):135-53. Epub 2015/06/20. doi: 10.1089/mab.2014.0096. PubMed PMID: 26090591.
23. Ravindranath MH, Terasaki PI, Maehara CY, Jucaud V, Kawakita S, Pham T, Yamashita W. Immunoglobulin (Ig)G purified from human sera mirrors intravenous Ig human leucocyte antigen (HLA) reactivity and recognizes one's own HLA types, but may be masked by Fab complementarity-determining region peptide in the native sera. Clin Exp Immunol. 2015;179(2):309-28. Epub 2014/09/10. doi: 10.1111/cei.12450. PubMed PMID: 25196542; PMCID: PMC4298408.
24. Ravindranath M, Jucaud V, Terasaki P. Immunobiology of Allograft Human Leukocyte Antigens in the New Microenvironment. SOJ Immunol. 2015;3(4):1-19.
25. Jucaud V, Ravindranath M, Terasaki P. Immunobiology of HLA class-Ib molecules in transplantation. SOJ Immunol. 2015;3(4):1-15.
26. Zhu D, Ravindranath MH, Terasaki PI, Miyazaki T, Pham T, Jucaud V. Suppression of allo-human leucocyte antigen (HLA) antibodies secreted by B memory cells in vitro: intravenous immunoglobulin (IVIg) versus a monoclonal anti-HLA-E IgG that mimics HLA-I reactivities of IVIg. Clin Exp Immunol. 2014;177(2):464-77. Epub 2014/03/13. doi: 10.1111/cei.12307. PubMed PMID: 24611451; PMCID: PMC4226597.
27. Sasaki T, Ravindranath MH, Terasaki PI, Freitas MC, Kawakita S, Jucaud V. Gastric cancer progression may involve a shift in HLA-E profile from an intact heterodimer to β2-microglobulin-free monomer. International Journal of Cancer. 2014;134(7):1558-70. doi: https://doi.org/10.1002/ijc.28484.
28. Ravindranath MH, Terasaki PI, Pham T, Jucaud V, Kawakita S. Suppression of blastogenesis and proliferation of activated CD4(+) T cells: intravenous immunoglobulin (IVIg) versus novel anti-human leucocyte antigen (HLA)-E monoclonal antibodies mimicking HLA-I reactivity of IVIg. Clin Exp Immunol. 2014;178(1):154-77. Epub 2014/06/04. doi: 10.1111/cei.12391. PubMed PMID: 24889882; PMCID: PMC4360205.
29. Ravindranath MH, Zhu D, Pham T, Jucaud V, Hopfield J, Kawakita S, Terasaki PI. Anti-HLA-E monoclonal antibodies reacting with HLA-la and lb alleles like IVIg as potential IVIg-immunomimetics: an evolving therapeutic concept. Clin Transpl. 2013:293-305. Epub 2013/01/01. PubMed PMID: 25095521.
30. Ravindranath MH, Terasaki PI, Pham T, Jucaud V, Kawakita S. Therapeutic preparations of IVIg contain naturally occurring anti–HLA-E antibodies that react with HLA-Ia (HLA-A/-B/-Cw) alleles. Blood. 2013;121(11):2013-28. doi: 10.1182/blood-2012-08-
